May 19, 2015 — Edwards Lifesciences announced it has temporarily halted its clinical trial of the Fortis mitral transcatheter heart valve. The company said that in consultation with trial investigators, it was decided to voluntarily implement a temporary pause on enrollment in its Fortis clinical program because of evidence of valve thrombosis. The company said the issue warrants additional investigation before restarting enrollment.
“We are working closely with the trial investigators and heart teams to gather additional information in this early study of transcatheter mitral valve replacement therapy,” the company said in a statement.
To date, the company said more than 20 patients globally have been implanted with the Fortis valve, all of whom had symptomatic mitral regurgitation and who were either compassionate cases or in one of their high-risk registries. While it is still very early in the program, the company anticipated that optimizing this therapy would be challenging and said it is continuing to learn which patients may benefit.
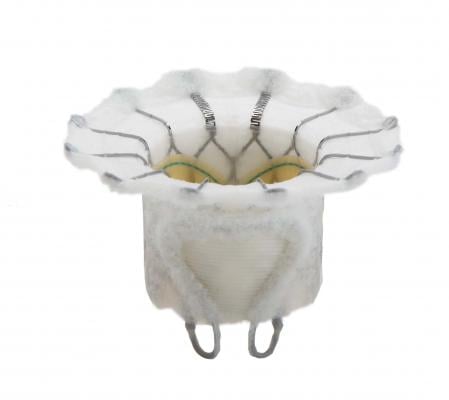
The Fortis transcatheter mitral valve features treated bovine pericardial tissue leaflets, a cloth-covered self-expanding frame designed to minimize pravalvular leak and an anatomical anchoring system. It is delivered using a transapical approach with a sheathless delivery system. The valve has paddles that fold out at the base and clip the native mitral valve leaflets to help anchor it. The first human trial began in August 2014 with centers in the United Kingdom, Canada and Switzerland.
The Fortis valve is not approved for sale in any country.
For more information: www.edwards.com


 December 24, 2025
December 24, 2025 









