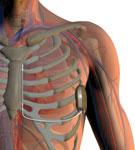
The S-ICD uses a lead that runs just under the skin, above the heart, eliminating the need for transvenous leads and securement inside the heart, which greatly simplifies the procedure and reduces possible complications.
The U.S. Food and Drug Administration (FDA) in October granted Boston Scientific Corp. regulatory approval for its S-ICD System, the world’s first commercially available subcutaneous implantable cardioverter defibrillator (S-ICD). The system sits entirely just below the skin without the need for implantable lead to be placed inside the heart. This offers patients an alternative to transvenous ICDs, which require leads to be placed in the heart itself.
“The S-ICD System establishes the first new category of cardiac rhythm management devices since the introduction of cardiac resynchronization therapy,” said Raul Weiss, M.D., associate professor of clinical and cardiovascular medicine at The Ohio State University in a press release. “Doctors now have a breakthrough treatment option that provides protection from sudden cardiac arrest without touching the heart.”
The S-ICD System was the star of the Heart Rhythm Society (HRS) annual meeting in May. Several experts at HRS said it is widely expected to revolutionize ICD implantation and greatly expand its implant base because of its simplied procedure and low complication rate. The booth of Cameron Health (developer of the technology) was packed with interested electrophysiologists (EPs), and several sessions discussed the device, including a late-breaking session on the U.S. pivotal trial.
In June, Boston Scientific finalized a deal to purchase Cameron Health and the S-ICD technology, which it expects to greatly boost its EP portfolio as the only subcutaneous ICD on the market. Boston Scientific expects to begin a phased launch of the S-ICD System that will expand over time as medical professionals are trained to implant it.
Simplified Implantation
A large number of patients in the United States who need ICDs do not receive the devices, partly because the implant procedures are invasive and require venous leads implanted in the heart. The S-ICD system will likely prove a more palatable option for these patients, said Martin Burke, D.O., FACC, FACOI, FRCP, professor of medicine, University of Chicago Hospitals, who is involved in the U.S. trial for the S-ICD system. Of the 13 percent of patients in the U.S. trial who had a previous ICD, only two opted for venous leads when offered the choice of the S-ICD system.
“It’s definitely a simpler implant than a venous implant,”
he explained.
An average venous lead implant procedure takes one to six hours depending on many variables, but an average S-ICD implant takes a very predictable one hour and 15 minutes, Burke said.
“The system has been very well accepted, the patients all love it,” Burke said. “Patients get this system, they like the fact that there is nothing in the heart.”
In addition to a simple installation, the programming of the device is also designed to be extremely easy to use, using one screen and having only three options.
European Experience
The S-ICD system was introduced in Europe in 2009 and more than 1,400 devices have been implanted in patients around the world. Pier D. Lambiase, M.D., Ph.D., FRCP, lead author, senior lecturer, honorary consultant cardiologist at Heart Hospital, University College of London, gained experience with the device in the United Kingdom and agrees the system is extremely easy to use.
“The learning curve is pretty steep. With two procedures you can get pretty proficient,” Lambiase said. “It will lower the threshold for implanting these devices.”
In addition, the easier, less invasive procedure is expected to greatly reduce complications and make lead extraction easier when it comes time for their regular replacement. Lambiase said there is no chance of heart valve damage and no possibility of venous perforation.
In the United Kingdom, Lambiase said the S-ICD so far has been shown to have the same cost equivalent as a traditional ICD. However, he explained the ease of the implant allows for increased procedure volume. He also expects, as a larger number of these devices are implanted, there will be a cost savings due to lower complication rates, especially lead infections.
Elimination of Fluoroscopy
Another advantage of the S-ICD device is its elimination of ionizing radiation to implant the leads, because the whole implant is done with direct visualization using anatomical landmarks.
“You don’t need fluoroscopy for this procedure,” Burke said. “I walked into the procedure room without lead on for the first time and I felt a little naked.”
Simple to Use, But Still the EP’s Domain
The simplicity of the S-ICD system has raised the question of whether ICDs can or should be implanted by cardiologists outside the specialty of electrophysiology. While the device is designed for ease of use, Burke warns that at this point only electrophysiologists should be implanting these devices. He said it is important during the initial phase of use in the United States to show the safety of the device with the use of experienced operators.
Indications for Use
The S-ICD System is intended to provide defibrillation therapy for the treatment of life-threatening ventricular tachyarrhythmias in patients who do not have symptomatic bradycardia, incessant ventricular tachycardia or spontaneous, frequently recurring ventricular tachycardia that is reliably terminated with antitachycardia pacing.
Video: How to Implant the S-ICD System
To view a short video presentation of how the S-ICD System is implanted, click here.
Video: Highlights of the S-ICD System U.S. Pivotal Trial
To see the highlights from the presentation of the S-ICD U.S. pivotal trial data at the Heart Rhythm Society (HRS) 2012 meeting, click here.



 January 13, 2026
January 13, 2026 









