March 15, 2016 — An independent panel of experts convened by the U.S. Food and Drug Administration (FDA) voted 9 to 0, with one abstention, that the benefits of Abbott's Absorb fully bioresorbable drug eluting coronary stent outweigh the risks. The device will now go to the FDA for a final decision on market approval, likely later this year. The FDA does not have to follow the recommendations of is advisory panels, but usually does.
(Update - July, 5, 2016 - FDA Approves First Totally Bioresorbable Stent)
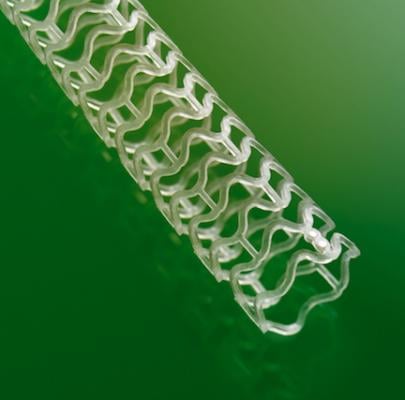
Absorb is a first-of-its-kind, fully bioresorbable stent for the treatment of coronary artery disease. While most stents are made of metal, the Absorb is made of dissolvable polylactic acid (PLA) plastic. It dissolves completely after two to three years, once it has done its job of keeping a clogged artery open and promoting healing of the artery. By contrast, metal stents are permanent implants that restrict vessel motion by caging the artery for the life of the patient.
Bioresorbable stents are seen by many cardiology experts as the next step in the evolution of stent technology. Many belive they may offer a pardigm shift is the standard of care, replacing metalic drug eluting stents (DES).
"In multiple randomized clinical trials, the Absorb bioresorbable vascular scaffold has demonstrated comparable outcomes to the leading permanent metallic stent. As a first-in-kind device with novel properties, including complete dissolution and natural restoration of vessel function, this is a remarkable achievement," said Gregg W. Stone, M.D., FACC, FSCAI, director, cardiovascular research and education, Center for Interventional Vascular Therapy, Columbia University Medical Center, New York-Presbyterian Hospital and the chairman of the ABSORB clinical trial program. "The available evidence supports an important role for this innovative device in the treatment of coronary artery disease."
The FDA panel also voted on the device's safety and efficacy as a treatment for coronary artery disease. On the question of whether there is reasonable assurance that the device is safe, the vote was 9 to 1 in favor. On the separate question of whether there is reasonable assurance that the device is efficacious, the vote was 10 to 0 in favor.
The panel also debated several key topics for the FDA to keep in mind as they consider approving the device for commercial use:
- Not approving the device for use in small vessels (<2.5 mm) due to increased stent thrombosis rates from thicker device struts than current DES;
- Updating the Absorb pre-procedure imaging approach to recommend online quantitative coronary angiography (QCA) reference vessel diameter (RVD) after routine RVD visual assessment of the vessel is found to be ≤3 mm;
- Revising Abbott’s proposed warning label to include the actual rates of stent thrombosis in small vessels;
- A wide non-inferiority margin for Absorb at 4.5 percent, based on retaining 50 percent of the treatment effect of BMS;
- Requiring post-dilatation in all cases when using Absorb, which will necessitate additional ancillary devices (and costs) during the procedure;
- Examining which patients should receive Absorb based on limited data on complex, ostial and bifurcation lesions, as well as lesions that require overlapping devices;
- Determining the appropriate amount of dual antiplatelet therapy (DAPT) for patients, since limited data was shown on Absorb patients who discontinued DAPT earlier than guidelines recommended; and
- Uncovering the clinical benefits of having the stent disappear over current metallic DES technologies, which are not yet determined with clinical data available to date.
The FDA advisory committee panel reviewed data from multiple studies of the Absorb dissolving stent, including ABSORB III, a company-sponsored U.S. clinical trial involving approximately 2,000 people that found the investigational device to be comparable to the market-leading metallic drug-eluting stent, Abbott's Xience drug eluting stent. At one year in ABSORB III, patients who received an Absorb dissolving stent had experienced comparable rates of specific adverse events — including heart disease-related death, heart attacks attributed to the stented artery and repeat procedures (collectively termed target lesion failure) — as compared to patients who received the Xience stent.
Read about the results of the ABSORB III Trial presented at TCT 2015.
Abbott submitted its pre-market approval (PMA) application for the Absorb dissolving stent in mid-2015 to the FDA, which convened today's advisory committee panel for expert advice on the device's clinical safety, efficacy, risk and benefit. The FDA routinely seeks input from advisory committees, especially for first-in-kind medical devices. The FDA's decision on Abbott's PMA for the Absorb dissolving stent is expected later this year.
The Society of Angiography and Cardiac Interventions (SCAI) issued a statement last fall supporting bioresorbable stent technologies. SCAI said initial data from trials was very encouraging and it looks forward to more long-term data over the next three to four years. Read the story.
"Fully dissolvable devices represent a transformative advance in the treatment of coronary artery blockages," said Charles Simonton, M.D., FACC, FSCAI, chief medical officer and divisional vice president of medical affairs for Abbott's vascular business. "The unique benefit of Absorb is that it opens the blockage like a metallic stent, but then goes away over time, allowing the artery to return to a more natural state. That makes the Absorb stent a very attractive option for many patients who don't want permanent implants inside their arteries for the rest of their lives. We thank the members of the panel for their thorough review of the data, and we look forward to continuing discussions with the FDA on our submission for approval of this device in the U.S."


 November 14, 2025
November 14, 2025 









