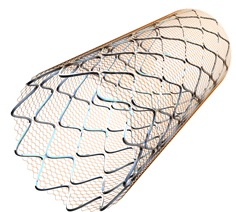
March 26, 2014 — Inspire MD Inc. has enrolled the first patient into the CARENET (CARotid Embolic protection study using microNET) multi-center European clinical trial for the new CGuard carotid embolic protection system.
The CARENET clinical study is a multi-specialty trial that is designed to provide data and evidence of the CGuard carotid embolic protection system to better understand the complexities and challenges of patients that experience carotid artery disease. The objective of the CARENET study is to evaluate the safety and efficacy of the CGuard system in the treatment of carotid lesions in consecutive patients suitable for carotid artery stenting (CAS).
The proprietary CGuard carotid embolic protection system uses the same MicroNet technology featured on the MGuard and MGuard Prime coronary embolic protection systems. The MicroNet technology is a single fiber knitted mesh wrapped on an open cell stent platform designed to trap debris that can travel downstream after a patient is treated with traditional stenting methods.
This technology seeks to protect patients from plaque debris and blood clots breaking off and traveling distally in the arteries, which can lead to life threatening strokes. The size, or aperture, of the MicroNet “pore” is only 150-180 microns in order to maximize protection against the potentially dangerous plaque and thrombus which is liberated during the procedure.
"I have treated many patients with carotid artery disease over the years, and I am excited to participate in the CARENET clinical trial using the unique CGuard embolic protection system," stated Professor Joachim Schofer, M.D., from the Hamburg University Cardiovascular Center, in Hamburg, Germany. "The small pore size of the MicroNet technology allows excellent blood flow while trapping potentially harmful plaque debris and thrombus. The CGuard technology provides an elegantly simple solution for embolic protection that has not been available in the past. I look forward to using this device with other sites throughout Europe in the CARENET trial to help treat my patients with carotid disease."
For more information: www.inspire-md.com


 January 05, 2026
January 05, 2026 









