October 31, 2013 — Tryton Medical Inc., a developer of
stents designed to treat bifurcation lesions, announced activities highlighting the latest data and experience with the Tryton Side Branch Stent at the 25th annual
Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium (TCT 2013).
Martin Leon, M.D. and principal investigator of the
study, presented the first results from the Tryton Side Branch Stent Pivotal IDE trial during the morning Late Breaking Clinical Trial session.
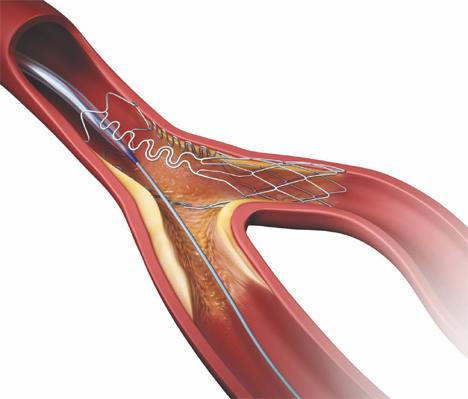
The Tryton Pivotal IDE trial was an international, randomized study that compares a Tryton Stent in the side branch to conventional provisional stenting (balloon angioplasty) in the side branch, with both study groups receiving a standard drug-eluting stent (DES) in the main vessel. The study, which is the first randomized U.S. Food and Drug Administration (FDA) Investigational Device Exemption (IDE) pivotal clinical trial to evaluate a dedicated bifurcation stent, enrolled 704 patients at 67 centers in North America and 11 countries throughout Europe and Israel. It is the largest coronary bifurcation study ever conducted and the first study to employ core lab angiographic (3-D and planar) and intravascular ultrasound (IVUS) analysis.
In advance of the unveiling of the results from this first-in-class trial, Leon presented Left Main and Bifurcation Stenting: An Advanced Operator’s Workshop during Session VIII on Dedicated Bifurcation Stents. He presented on the topic of “The Case for Dedicated Bifurcation Stents; Device and Regulatory Landscape; and Anticipation of the Pivotal Tryton Results.”
Leon and Patrick Serruys, M.D. and Ph.D., chaired a symposium regarding the Tryton Side Branch Stent. At the symposium, clinicians further examined the clinical and angiographic outcomes from the IDE trial, reviewed the historic body of clinical evidence, presentred real-world Tryton Stent cases and provided perspectives on treating a broad spectrum of bifurcation anatomies.
Additionally, the Tryton Stent was featured in the Challenging Cases forum and in Poster Abstract sessions:
- Robin Kraak, M.D., hosted: “Treatment of complex coronary bifurcation lesion; ABSORB BVS in combination with Tryton short dedicated bifurcation stent” and “Treatment of complex coronary bifurcation lesion in a patient with acute ST elevation myocardial infarction; ABSORB BVS in combination with Tryton dedicated bifurcation stent.”
- Balazs Berta, M.D., hosted “Trifurcational occlusion treated by dedicated bifurcation stent in ST-elevation myocardial infarction from transradial approach.”
- Maik Grundeken, M.D., hosted “First Report on Long-term Clinical Results After Treatment of Coronary Bifurcation Lesions With the Tryton Dedicated Bifurcation Stent.”
For more information: www.trytonmedical.com


 January 05, 2026
January 05, 2026 









