December 6, 2013 — HeartWare International Inc. has acquired CircuLite Inc., a developer of the Synergy Circulatory Support System, which is designed to treat less sick, ambulatory, chronic
heart failure patients who are not yet inotrope-dependent.
According to the terms of the merger agreement, HeartWare has acquired all of the issued and outstanding equity interests of CircuLite for consideration of $30 million, consisting of approximately $18 million in HeartWare common stock and cash of approximately $12 million to settle CircuLite's debt and transaction expenses, plus certain contingent success payments due upon satisfaction of regulatory and commercial milestones not to exceed $320 million in the aggregate over a 10-year period.
"CircuLite has pioneered the partial-assist approach and demonstrated that this technique can significantly enhance the quality of life for this group of patients, which is believed to be a substantially larger population than the end-stage heart failure patients that HeartWare currently treats with our full-support
Ventricular Assist Devices [VADs],” said Doug Godshall, president and CEO, HeartWare. “CircuLite's next generation endovascular system, which will be implanted collaboratively by cardiologists and surgeons in a hybrid cath lab setting, offers an extremely compelling interventional approach to circulatory support. While our HVAD and MVAD Systems offer minimally invasive treatment to end-stage heart failure patients, the Synergy platform offers even less invasive and ultimately interventional options to earlier-stage heart failure patients."
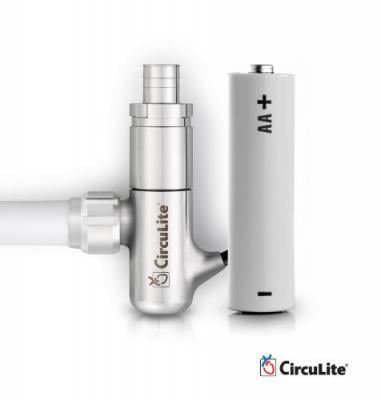
The Synergy Surgical System, which received CE marking in the European Union in 2012, is designed for long-term support and is intended to reduce the heart's workload while improving blood flow to vital organs. Approximately the size and weight of a AA battery, the CE-marked Synergy Surgical System is implanted through a right, mini-thoracotomy procedure and does not require a sternotomy or cardiopulmonary bypass. With this approach, the inflow cannula is placed in the left atrium, and the outflow graft is attached to the subclavian artery. CircuLite's proprietary micro-pump is then placed in a pacemaker-like pocket and attached to the inflow cannula and outflow graft, which connects to a wearable, external controller and battery pack.
The system is currently undergoing an upgrade to resolve issues that arose post commercial release. Surgical System sales are expected to resume in a controlled fashion following regulatory approval to re-launch the system in Europe and will focus on building experience at a small number of centers to refine training techniques and implement additional system upgrades in advance of a full rollout.
"The Synergy system is a novel entrant in the partial-support space for treating patients with earlier stage heart failure," said Martin Strueber, M.D. University Heart Center Leipzig, Germany. "To date, there is no viable option for those patients who have used biventricular pacemakers without success and who are not yet sick enough for a VAD or cardiac transplantation. Having implanted Synergy systems and witnessed the marked benefits to my patients and increased referrals I received for both full and partial mechanical support, I look forward to technological advancements to the system and a restored availability for patients. Longer-term, the endovascular approach, which we have explored extensively in Leipzig in partnership with our cardiology colleagues, is truly exciting and holds considerable promise."
"We are confident that HeartWare's technical, regulatory, sales and marketing capabilities will have a profound impact on enabling the Synergy system to reach its full potential," said Daniel Burkhoff, M.D., Ph.D., chief medical officer, CircuLite. "There is considerable opportunity for the current Synergy system, and through continued investment and innovation, we believe we could expand the circulatory support market with the launch of a groundbreaking, endovascular treatment for patients with earlier stage heart failure."
"The team at CircuLite created the Synergy Endovascular System in collaboration with several leading cardiologists and received approval to commence a feasibility study earlier this year,” said Godshall. “We look forward to commencing an investigation of this elegant device, as we believe it has the potential to vastly expand the mechanical support market by bringing cardiologists into the implant procedure for the first time.”
Under the terms of the merger agreement, CircuLite security holders may be entitled to receive additional clinical and commercial success payments upon achievement of specified regulatory and revenue-based milestones.
Potential regulatory milestone payments include:
- $20 million payable in HeartWare common stock upon full European re-launch of the Synergy Surgical System
- Up to $75 million payable in cash or HeartWare common stock upon CE mark of the Synergy Endovascular System
- $50 million payable in cash or HeartWare common stock upon U.S. Food and Drug Administration (FDA) Pre-Market Application approval of the Synergy Endovascular System.
Potential revenue-based success payments include:
- Up to $15 million payable in cash or HeartWare common stock when the 12-month trailing sales of the Synergy Surgical System reach $30 million
- $85 million payable in cash or HeartWare common stock upon achievement of the 12-month trailing sales of $250 million of Synergy Systems (surgical or endovascular)
- Up to $75 million (subject to adjustment) in royalties on sales of surgical and endovascular CircuLite products payable in cash or HeartWare common stock.
The total upfront and earnout consideration is subject to certain performance criteria and cannot exceed $350 million. All consideration may only be earned through the eighth anniversary of the closing of the transaction, with the exception of the milestone payment based on trailing sales of $250 million, which may be earned through the 10th anniversary. Any payments made in HeartWare common stock, including the upfront consideration, are calculated using a 60-day volume weighted average price.
This transaction was approved by the board of directors of HeartWare and board of directors and shareholders of CircuLite.
For more information: www.heartware.com, www.circulite.net


 June 19, 2024
June 19, 2024 









