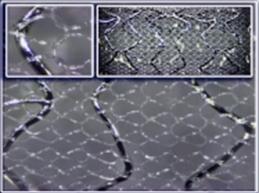
May 24, 2013 — InspireMD Inc. announced new six-month results from the MASTER (MGuard for Acute ST Elevation Reperfusion) trial demonstrating that the MGuard embolic protection stent (EPS) outperformed bare metal stents and drug-eluting stents in all-cause mortality in ST-segment elevation myocardial infarction (STEMI) patients. With its micro-net mesh sleeve, MGuard EPS prevents unstable arterial plaque and thrombus (clots) that cause heart attack blockage from breaking off and exacerbating damage.
The MASTER trial achieved its primary endpoint (p value = 0.008), in complete ST-segment resolution at 60 to 90 minutes post-procedure (a strong predictor of mortality). Secondary endpoint clinical outcomes continue to show a lower mortality rate with MGuard EPS compared to control (0.5 percent vs. 2.8 percent, P=0.06) at six months. These findings corroborate the previously announced 30-day results showing that all-cause mortality with MGuard EPS was lower than bare metal and drug-eluting stents used as a control (0 percent vs. 1.9 percent, P=0.06).
In the MASTER trial, a total of 433 patients with STEMI presenting within 12 hours of symptom onset undergoing percutaneous coronary intervention (PCI) were randomized at 50 sites in nine countries to the MGuard EPS (n = 217) or commercially available bare metal or drug-eluting stents (n = 216).
Watch the video on InspireMD’s Micronet Technology at www.youtube.com/watch?v=EbrhcQMM7YE.
For more information: www.InspireMD.com


 January 05, 2026
January 05, 2026 









