February 15, 2012 — Medtronic Inc. announced the start of two clinical initiatives evaluating the broader, real-world clinical use of the company’s Symplicity renal denervation system across multiple conditions. Furthering its leadership in the development of renal denervation therapy, Medtronic launched the Global Symplicity patient registry, which will evaluate the real-world, long-term impact of renal denervation in more than 5,000 patients, as well as SYMPLICITY-HF, the first clinical trial to examine renal denervation in patients with chronic heart failure and renal impairment.
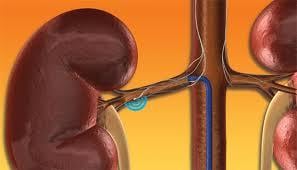
Renal denervation therapy is a minimally invasive, catheter-based procedure that modulates the output of nerves that line the walls of the arteries leading to the kidneys. These nerves impact the sympathetic nervous system, which affects the major organs that are responsible for regulating blood pressure: the brain, heart, kidneys and blood vessels.
“These research initiatives represent part of Medtronic’s broad commitment to partner with the medical community to explore the use of renal denervation in a number of disease states characterized by hyperactive sympathetic nervous system drive,” said Sean Salmon, senior vice president and president of coronary and renal denervation, Medtronic. “Data from the Global Symplicity Registry and the SYMPLICITY-HF clinical trial will build upon the substantial renal denervation data Medtronic has generated in patients with treatment-resistant hypertension to date.”
For more information: www.symplifybptrial.com, www.medtronic.com


 January 05, 2026
January 05, 2026 









