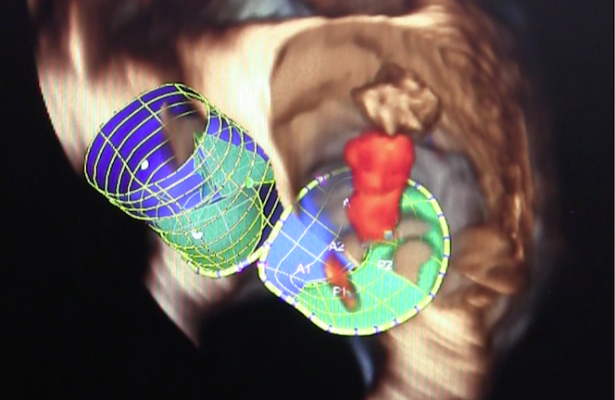
April 24, 2015 — Minneapolis Heart Institute Foundation (MHIF) physicians are conducting a research study using the first transcatheter mitral valve replacement (TMVR) in the United States at Minneapolis Heart Institute at Abbott Northwestern Hospital.
Wes Pedersen, M.D., principal investigator in the study said, “The Tendyne Bioprosthetic Mitral Valve is designed to give implanting physicians total control because it is fully repositionable and retrievable, which allows physicians to see the outcome before the procedure is closed.” If proven efficacious, this may be an option for patients with diseased, damaged or malfunctioning mitral valves who are not deemed candidates for conventional surgery.
“The transcatheter procedure involves implanting the replacement valve inside a beating heart without the need for open heart surgery or cardiopulmonary bypass. Through a small incision in the chest, a catheter enters the bottom of the heart and travels into the left ventricle to position the replacement valve within the natural mitral valve,” said Robert Saeid Farivar, M.D., Ph.D., chief cardiothoracic surgeon and co-principal investigator in the study.
Securing the research study in the U.S. this quickly, following Australian and United Kingdom studies, helps keep the United States at the forefront of emerging transcatheter valve therapy research. In September 2013, the U.S. Food and Drug Administration (FDA) issued guidance on Investigational Device Exemptions (IDEs) for early feasibility medical device clinical studies to encourage this type of pivotal research here in the U.S. Per the FDA guidance, IDE’s offer a unique opportunity to obtain clinical experience with a new or modified device or new clinical use while utilizing appropriate subject protection measures and good clinical study practices. Pedersen said, “Collaborating and learning together across research, technology and regulatory improves our ability to identify potential innovative treatments and better patient outcomes here in the U.S. This first study implant has occurred, the participant is ready for discharge and we couldn’t be more thrilled.”
The Tendyne TMVR is a fully retrievable and repositionable, apically tethered tri-leaflet porcine pericardial valve sewn onto a nitinol frame that was specifically designed to address the complex mitral anatomy of functional, degenerative and mixed etiology mitral regurgitation.
For more information: www.mplsheart.org


 January 05, 2026
January 05, 2026 









