February 18, 2014 — Neovasc Inc. announced that a first-in-human implantation of its Tiara transcatheter mitral valve was successfully performed Jan. 30 by physicians at St. Paul’s Hospital in Vancouver, BC. The transapical procedure resulted in the elimination of mitral regurgitation (MR) and significantly improved heart function in the patient, without the need for cardiac bypass support and with no procedural complications.
The implantation was conducted by Anson Cheung, professor of surgery and director of cardiac transplant, and John Webb, director of interventional cardiology at St. Paul ’s Hospital. They were supported by Stefan Verheye, senior interventional cardiologist at the Antwerp Cardiovascular Center / ZNA Middelheim, Belgium and Shmuel Banai, medical director of Neovasc and director of interventional cardiology at Tel Aviv Medical Center, Israel.
“This 73 year-old male patient had severe functional mitral regurgitation and was considered an extremely high risk candidate for conventional valve repair or replacement surgery,” Cheung said. “The transapical implantation of the Tiara valve was completed quickly and without complications. It resulted in a well-functioning bioprosthetic valve with no significant paravalvular leak or residual MR.”
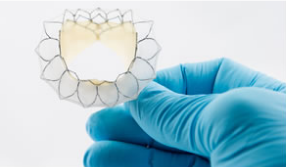
Tiara is a self-expanding mitral bioprosthesis specifically designed to treat mitral valve regurgitation, a serious and poorly served condition that requires development of highly specialized devices to address the complex mitral anatomy. Significant mitral regurgitation can lead to heart failure and death. Conventional surgical treatments are only appropriate for about half of the estimated four million MR patients in the United States.
Tiara is implanted in the heart using a minimally-invasive, transcatheter approach and is designed to replace the diseased native mitral valve without the need for open heart surgery or use of a cardiac bypass machine.
For more information: www.neovasc.com


 January 05, 2026
January 05, 2026 









