
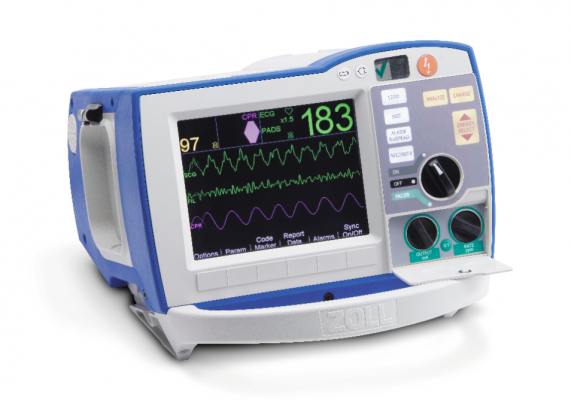
For U.S. hospitals and emergency medical services (EMS) looking to upgrade their aging defibrillator-monitors, new technologies added to these systems enable more feedback to first responders and can help speed door-to-balloon times by transmitting pre-hospital ECGs.
Market Overview
There are only three vendors left in the U.S. market that sell U.S. Food and Drug Administration (FDA) cleared defibrillator-monitors — Physio-Control, Zoll and Philips Healthcare. All three vendors’ systems offer similar operation and similar features. Here are a few features prospective buyers may consider when reviewing which systems to purchase.
Improved CPR Monitoring
Chest compressions during cardiopulmonary resuscitation (CPR) can greatly vary based on the strength and experience of the person delivering CPR, the patient’s body habitus and whether compressions are delivered on the floor, a bed, table or the back of a moving ambulance. The 2010 American Heart Association (AHA) Guidelines place a heavy emphasis on quality CPR, but without a built-in CPR sensor, it is difficult to judge how hard clinicians are pushing. This variability has led vendors to add software to monitor chest compressions.
The feature delivers instant audio-visual feedback of compression depth and rate, chest recoil, hands-off time and the ventilation rate. The systems also store this data for later review.
Zoll’s R Series and Philips HeartSmart MRx both offer CPR feedback. Zoll also provides a CPR artifact filter on the ECG waveforms. Physio-Control’s Lifepak 15 and 20e and Zoll’s R Series offer a CPR metronome to help responders keep pace with the AHA guideline rate of 100 compressions per minute. The Lifepak 20e also offers cprMAX technology, giving the option to provide a specified CPR interval before delivering the first shock.
Transmission of Pre-Hospital ECGs
Some of the systems offer the ability for EMS to remotely transmit ECGs from the defibrillator-monitor in the field to a hospital ahead of patient arrival. These waveforms are typically transmitted via a Wi-Fi/cellular modem or Bluetooth enabled cell phone to a software system in the emergency department (ED). From there, the waveforms can be evaluated by an ED physician, or sent to a cardiologists smartphone or tablet device.
If a ST-elevated myocardial infarction (STEMI) is diagnosed, it can enable earlier activation of the cath lab, including calling in staff for late-night cases prior to patient arrival. Some hospitals use these STEMI-alert systems to bypass the ED, transporting the patient directly to the cath lab.
This feature is available on the Philips HeartSmart MRx and Physio-Control Lifepak 15 and 20e systems. Zoll offers its RescueNet 12-Lead free transmission system that allows healthcare providers to receive and manage 12-lead ECGs via encrypted e-mail.
Automatic Diagnostics
The latest defibrillator-monitors offer several automated features to speed diagnosis of problems with the system, which may be overlooked in an emergency, and to speed diagnosis of the patient’s cardiac condition.
All three vendors offer lead-fault indicators, where the system will indicate there is an issue with a lead. Some systems can also indicate if the pacer is not capturing data or if it is not pacing.
The software of these systems has become clinically sophisticated and can be configured to detect and sound alarms for high or low heart rates, asystole, ventricular fibrillation, ventricular tachycardia, extreme tachycardia, extreme bradycardia, premature ventricular contraction (PVC) rate and ST-segment elevation.
In addition, most systems will suggest a diagnosis for STEMI when detected in the waveforms. The Philips’ HeartSmart MRx offers STEMI culprit artery identification based on ECG data.
Some of these systems also perform automated self-diagnostics each day to check their readiness status.
Pediatric Specific Features
There has been a push in the past decade for pediatric specific CPR guidelines and use of pediatric specific medical devices, rather than using technology designed for larger adult patients. Today’s defibrillator-monitors offer these features. All vendors offer a pediatric specific paddle feature. Philips offers slide-off adult adapters to expose pediatric electrodes underneath.
Zoll offers OneStep pediatric CPR electrodes, which were the first electrodes on the market with a built-in sensor that reports CPR quality on young children up to age 8. In the event of a cardiac arrest, as soon as the pediatric electrodes are placed on the patient, the R Series immediately adjusts its AED analysis algorithm to pediatric parameters in order to reduce the likelihood that a compensating rhythm will be terminated. This ensures that any unique pediatric ECG morphology will be accurately identified when a shock is indicated.
The pediatric electrodes will also automate the R Series to reduce shock energy to a starting dose of 50 Joules.
Parameters Monitored
Each system has varied monitoring options beyond ECG, which is a point of difference with some systems. In addition to ECG monitoring, some systems offer blood oxygen saturation (SpO2 or pulse oximetry), end-tidal carbon dioxide concentration in the expired air (ETCO2 or capnography), noninvasive and/or invasive blood pressure monitoring and patient temperature. SpO2 and ETCO2 features are usually offered through a partnership with another vendor that specializes in these monitoring technologies.
Connectivity Considerations
There is currently no industry agreed upon format for ECG waveforms, resulting in numerous integration issues when ECG waveform files are sent to electronic medical records, cardiovascular PACS or ECG management systems. Defibrillator-monitors primarily use XML and PDF formats, so it might be wise to ask for input from the hospital’s IT support team to get feedback on how this data can be integrated into the IT systems you have.
Comparison Chart
This article ran as an introduction to the 2014 comparison chart of defibrillator-monitor systems. To access the chart go to www.dicardiology.com/content/defibrillator-monitors, and create a free login. The login only takes a minute. Vendors that offer these system in the United States include:
Philips Healthcare
www.healthcare.philips.com
Physio-Control
www.physio-control.com
Zoll Medical Corp.
www.zoll.com



 November 12, 2025
November 12, 2025 









