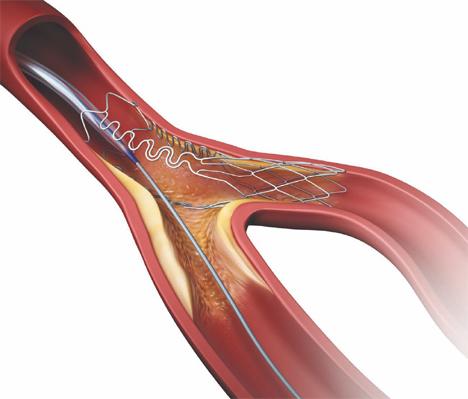
The Tryton Side Branch Stent.
Stenting of lesions at coronary vessel bifurcations is one of the most challenging percutaneous interventions, along with a high risk of procedural complications and restenosis. Several companies are developing specialized bifurcation stents to help make the job easier. Currently there are no dedicated bifurcation stents with FDA clearance.
(March 2017 UPDATE - read the article "FDA Clears First Dedicated Coronary Bifurcation Stent.")
About 15 percent of PCI cases involve bifurcations, which roughly equals between 200,000-300,000 patients a year, said Alexandra Lansky, M.D., FACC, director of clinical services, center for interventional vascular therapy, New York-Presbyterian Hospital/Columbia University Medical Center, director of the angiographic core analysis laboratories and director of the Women’s Cardiovascular Health Initiative at the Cardiovascular Research Foundation. Bifurcations are not only complicated by their morphology, but also by the fact they are more prone to thrombosis, restenosis and target lesion revascularization.
She said current bifurcation stenting methods include the crush, culotte and T-stenting techniques, which use existing FDA-cleared stents. The crush involves deploying the side-branch stent with a portion extending into the main vessel, which is “crushed” against the sidewall of the vessel when the main artery stent is inflated. The culotte method, also referred to as the “kissing stent” or “kissing balloon” technique, involves deploying two stents in the main and side branch vessels and inflating them simultaneously so they compress into each other. The end result on fluoroscopy looks like a pair of pants, or culotte in French. T-stenting involves a stent in both the main and side branch and expanding a balloon wired through the main branch stent struts to create a lumen opening into the side branch.
These two-stent methods all distort the metal bodies of the stents, causing problems with apposition and turbulence in the vessels, both of which Dr. Lansky said are both linked to restenosis. The crush technique also causes multiple layers of DES stent struts against the lumen, which has been associated with much higher rates of stent thrombosis.
Another option is to only stent the main vessel and leave access to the side branch in case further intervention is needed, which is referred to as the provisional approach. She said three key studies – Nordic, BBC ONE, and CACTUS – evaluated the best techniques for stenting these lesions. “All of these studies showed the same thing – the best approach is to only stent the main vessel using the provisional approach,” Dr. Lansky said. None of the studies found a significant advantage in stenting the side branch vessel unless there was a compelling reason, such as 70 percent or more stenosis. Dr. Lansky said the studies all found higher restenosis rates associated with stenting both vessels.
New Stent Technology
“There is a need for a dedicated side branch stent, but it must be easy to use,” said Dr. John Ormiston, Auckland City Hospital and Mercy Angiography, Auckland, New Zealand, and serves on advisory boards for Devax, Abbott Vascular, and Boston Scientific, each of which is developing bifurcation stents.
He presented at ACC 2009 on the status of eight bifurcation stents in development. These include the Axxess stent by Devax, Boston Scientific’s TAXUS Petal, Minvasys’ Nile Croco, Medtronic’s dedicated bifurcation stent, Abbott’s Pathfinder, TriReme Medical‘s Antares II, Capella’s Sideguard, Stentys, and Tryon Medical’s Tryton Side Branch Stent. Y-Medical is also developing the Sidekick delivery system to allow side branch stents to be torqued into alignment.
Ormiston said many of these devices take different approaches. He said Stentys created a self-expanding nitinol stent with struts designed to be separated, making it much easier to make openings with a balloon to access the side branch. Medtronic’s stent uses two wires to snug it into position. He said Abbott’s system is designed to prevent the two deployment wires from wrapping and binding. The Antares has a torquable shaft to better position it.
AXXESS
Of these devices, Drs. Ormiston and Lansky said the Axxess is the furthest along in development and will likely be the first to market, followed by Tryton. Dr. Lansky said Devax has filed for CE mark approval. In June the FDA conditionally approved an investigational device exemption (IDE) for the AXXESS Biolimus A9-Eluting Bifurcation Stent System, allowing the company to initiate a pivotal clinical trial (DIVERGE II) in the U.S. The multicenter, blinded, controlled, randomized trial will compare the treatment of bifurcation lesions with the AXXESS stent to standard techniques with conventional stents. The principal investigator is Jeffrey Moses, M.D., professor of medicine and director of the Center for Intravascular Therapy at Columbia University Medical Center in New York.
Both Drs. Lansky and Ormiston work as investigators for the Drug-Eluting Stent Intervention for Treating Side Branches Effectively (DIVERGE) trial, which is testing the effectiveness and safety of the Axxess stent. Devax has already implanted more than 430 AXXESS stents in two clinical studies conducted outside the U.S. So far Dr. Lansky said the trial has shown restenosis and revascularization rates that are much lower than using existing stenting methods.
The Devax is a self-expanding, nitinol, main branch stent with a built-in bifurcation in the stent struts Dr. Lansky said the stent is skirt-shaped to fit in the area where the area where the vessel splits. The stent has gold markers on the ends of its structure to clearly show where the stent struts start and end, as well as to help when deploying additional stents in the main and side branches. She said the earliest the FDA might consider device approval would be around 2011.
Tryton
Dr. Lansky said Tryton is starting to plan for its FDA pivotal trial, but does not yet have FDA IDE trial approval. The Tryton Side Branch Stent is deployed in the side branch artery using a standard single-wire, balloon-expandable stent delivery system. When expanded it has a conventional strut scaffold in the side branch, but the struts trail off into three thin bands that narrow down to a single strut in the primary vessel. A conventional drug-eluting stent is then placed in the main vessel. The stent demonstrated excellent initial clinical results and is available for sale in Europe, where it received CE mark in 2008.
VIDEO: Demonstration of the Tryton Dedicated Bifurcation Stent
Antares
TriReme Medical’s Antares Coronary Stent System received CE mark for European sales last October. It is a thin strut (0.0035 inch) stainless steel stent with progressive surface finish. It permits deployment at or near bifurcation lesions with a single balloon inflation. It maintains access to the side branch throughout the procedure, so recrossing with another wire is not required. The stent has a radio-opaque side branch stabilizing wire. There are also radiopaque markers built into the stent struts that are expanded into the side branch for better visualization and stent positioning. The company said these markers could also be used if a second stent is deployed in the side branch.
TAXUS Petal
“Bifurcation stenting is really an unmet need,” said Dennis Fiedler, Boston Scientific’s vice president in research and development. When Boston Scientific speaks with interventional cardiologists, bifurcation stents are always on the top of their want lists. “What they want is to treat that main branch and be able to treat the side branch if needed.”
Boston purchased ASI a few years ago, which provided the company with bifurcation stent technology that has been developed into the TAXUS Petal drug-eluting stent prototype. The stent has an opening on the side for the side branch vessel. When expanded it opens radio-opaque “petals” about 1-2 mm into the side branch, providing drug-elution around the ostium. Fiedler also said the petals make it easier to visualize the area and allow for stent overlap if a side branch stent is deployed.
Fiedler said the stent was evaluated in the Petal first-in-man trial by doctors Eberhard Grube in Germany, John Ormiston in New Zealand and Thierry Lefevre in France. They offered improvement suggestions for better alignment to make the stent easier to deliver. The catheter is now being redesigned and Fiedler hopes a second in-man evaluation can take place by the same doctors in 2010. If successful, Fiedler said these studies will help lay the groundwork to seek CE mark approval in Europe, possibly by 2011 or 2012.
Pathfinder
Abbott put its bifurcation stent program on hold to devote more resources to getting XIENCE to market, but is now revisiting the Pathfinder. Dr. Chuck Simonton, Abbott’s chief medical officer and division vice president of medical science, said the company hopes to find a faster path for FDA approval and wants to use a modified XIENCE platform as a dedicated side branch stent. He said the company hopes to begin its first-in-man clinical trial in Europe in the first half of 2010.
Medtronic
Medtronic said its Y-shaped dedicated bifurcation stent is currently being tested in the 60-patient BRANCH study in Australia and New Zealand. The first patient was implanted in February 2008. The six-month data will be presented in a session at TCT 2009. The stent is delivered over a dual-wire delivery system through a single catheter.
The morphology is different in all patients, particularly with the flaring and angles of the side branch ostium, so there is no such thing as a one-size fits all solution in bifurcations, which leaves the door open to a number of solutions manufacturers are proposing. <
For More Information:
www.devax.net
www.trytonmedical.com
www.trirememedical.com
www.abbott.com
www.bostonscientific.com




 November 24, 2025
November 24, 2025 









