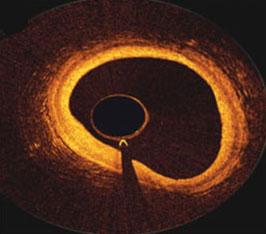
September 26, 2014 — The first randomized trial to examine serial optical coherence tomography (OCT) in primary percutaneous coronary intervention (PCI) was reported at the Transcatheter Cardiovascular Therapeutics (TCT) scientific symposium last week. The trial showed similar outcomes in to a control group that did not use OCT. The nine-month follow up data showed significantly smaller area stenosis and a trend toward fewer uncovered struts in the OCT guided group.
OCT uses light emitted from an intravascular catheter to capture high-resolution cross sectional imaging from within coronary arteries. OCT STEMI is the first randomized multicenter study to examine routine use of OCT guidance during stent implantation in patients with ST-elevation myocardial infarction (STEMI), the most serious form of a heart attack) undergoing primary PCI. The primary endpoints were the rate of major adverse coronary events (MACE) including death, myocardial infarction, and ischemia-driven target lesion revascularization at 30 days and nine months. The number of uncovered struts, area stenosis and minimal lumen diameter were assessed by OCT after nine months.
A total of 201 patients were enrolled in the study and pre-treated with aspirin, heparin and clopidogrel. After radial diagnostic angiography, patients were randomly assigned to either primary PCI alone (n=96) or to primary PCI with OCT guidance (n=105). Drug-eluting stents (DES) were used in this trial and dual antiplatelet therapy was recommended for 12 months in both groups.
The rate of MACE was very low and comparable at 30 days and nine-month follow up in both groups. The rates of stent thrombosis, late lumen loss and binary restenosis were also similarly low in both groups. Nine-month OCT analysis revealed significantly smaller area stenosis (p=0.001) and a trend toward fewer uncovered struts (p=0.07) in the OCT guided group.
Baseline demographic and procedural characteristics were well balanced in both groups. Based on the OCT data, more stents were used in the OCT group (likely based upon the intraprocedural OCT findings), and fluoroscopy time was significantly longer in the OCT group.
“The OCT STEMI trial demonstrates the potential merit of OCT guidance during drug-eluting stent implantation in primary PCI,” said lead investigator Pavel Cervinka, Ph.D., M.D. Cervinka is a professor from Faculty Hospital Hradee Králové and Masaryk Hospital in the Czech Republic. “The study also indicates that the procedure is safe for STEMI patients, and larger randomized trials with longer-term follow-up are warranted.”
The OCT STEMI trial was funded by grants from Boston Scientific and A Care. Cervinka reported no disclosures.
For more information: www.crf.org, www.tctconference.com