March 25, 2012 — New evidence shows that with appropriate preparation, angioplasty can be safely and effectively performed at community hospitals without on-site cardiac surgery units. This was according to data presented from the CPORT-E trial during the American College of Cardiology's (ACC) 61st Annual Scientific Session this week in Chicago.
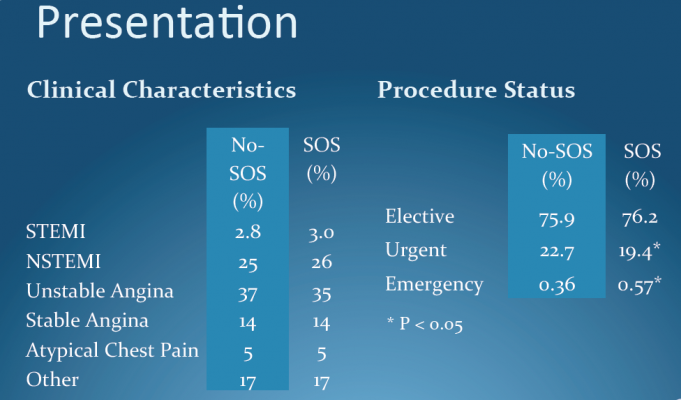
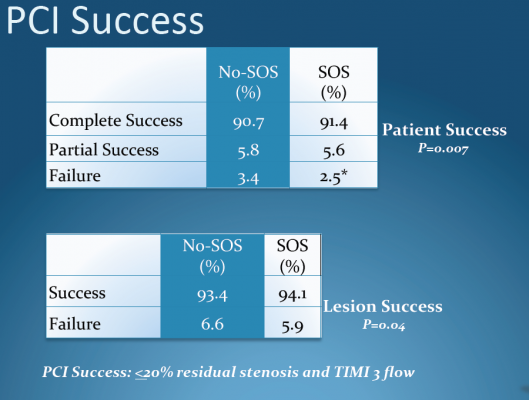
The study is the first randomized controlled trial to investigate elective cath lab angioplasty (or percutaneous coronary intervention, which includes stenting and balloon angioplasty) in community hospitals in the United States. Results showed no difference in death rates among patients undergoing elective angioplasty at facilities with and without on-site cardiac surgery units. There were also no significant differences in rates of complications such as bleeding, renal failure and stroke.
“The study shows that under certain circumstances, non-primary angioplasty can be performed safely and effectively at hospitals without on-site cardiac surgery,” said Thomas Aversano, M.D., associate professor of cardiology at Johns Hopkins University and the study’s lead investigator.
Until a recent guideline change by the American College of Cardiology and the American Heart Association, community hospitals without cardiac surgery units performed only emergency angioplasties. Patients needing elective angioplasty were transferred to facilities with on-site cardiac surgery units.
“The study supports and reinforces the [new] guidelines,” said Aversano, adding that the findings can help hospitals and healthcare planners more efficiently allocate financial and human resources.
The ability for community hospitals to offer elective angioplasty benefits patients, Aversano said. Other studies have shown that patients are often reluctant to transfer to a hospital that may be farther away or more expensive than their community hospital. “It’s not just a question of patient convenience — it’s also a question of access,” he said.
The study randomly assigned 18,867 patients to receive elective angioplasty either at a facility with on-site cardiac surgery or at one of 60 community hospitals that had undergone special preparations to perform the procedure. The researchers tracked patient outcomes for nine months.
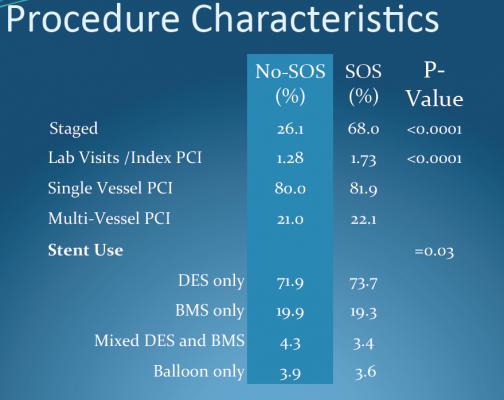
To participate in the study, community hospitals were required to complete a formal angioplasty development program to prepare their staff and establish policies and protocols in what Aversano called a “system-wide approach.”
The community hospitals also had to demonstrate a capacity to perform at least 200 angioplasties per year. For most participating hospitals, meeting the study requirements took significant effort, as hospitals ramped up from providing a low volume of emergency angioplasties — or no angioplasties at all — to supporting frequent elective angioplasties.
“The thing I found most impressive was the degree of dedication from the staff at these community hospitals,” Aversano said. “All of them were incredibly dedicated to the success of this program, to their hospital and to the care of the people in their community. It was quite remarkable.”
This study was self-funded by the sites participating in the trial. Aversano reported no conflicts of interest.
To see the video interview with Aversano at ACC.12, click here.
For more information: www.acc.org


 January 05, 2026
January 05, 2026 









