September 3, 2013 — St. Jude Medical Inc. announced the CE mark approval of its next-generation EnligHTN renal denervation system for treating patients with drug-resistant, uncontrolled hypertension. The system features an advanced generator that delivers simultaneous ablations via a multi-electrode catheter, reducing total ablation time with the new EnligHTN system by more than 80 percent in comparison to the first generation system — from approximately 24 minutes to four minutes. The EnligHTN system was display during the 2013 European Society of Cardiology (ESC) meeting Aug. 31 to Sept. 3.
“The new EnligHTN system improves the procedure by significantly reducing ablation time and providing optimized ablation monitoring feedback,” said Thomas Lüscher, professor and chairman of cardiology, Cardiovascular Center at the University Hospital in Zurich, and editor-in-chief of the European Heart Journal. “Renal denervation is a relatively simple, minimally invasive procedure that is emerging as an important new avenue for managing hypertensive patients who do not respond to medications.”
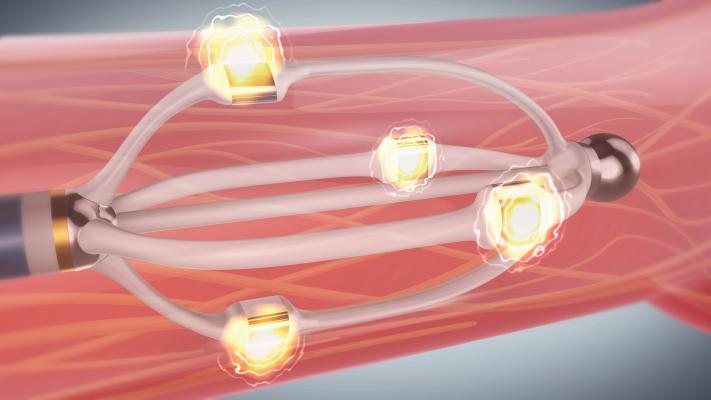
Renal denervation is a catheter-based ablation procedure used to treat patients with drug-resistant hypertension (high blood pressure). The EnligHTN system delivers radiofrequency (RF) energy from an ablation catheter to create lesions along the renal nerves, a network of nerves in the walls of the renal arteries thought to help control blood pressure. The intentional disruption of the nerve supply has been clinically found to help reduce systolic and diastolic blood pressure. The system catheter has a unique, non-occlusive basket design, helping physicians deliver a predictable treatment pattern while allowing for continuous blood flow to the kidney throughout the procedure.
Blood pressure reduction is quickly achieved by simultaneously administering 60-second ablations from all four catheter electrodes, which is typically performed two times in each renal artery. The advanced generator also has an icon-based touch-screen interface that allows physicians to easily view and record procedure information.
“The next generation EnligHTN system offers physicians our proven multi-electrode catheter with a new intuitive, faster generator that quickly and effectively delivers consistent ablations with a significant reduction in procedure time,” said Frank J. Callaghan, president of the St. Jude Medical cardiovascular and ablation technologies division. “These advancements deliver on our strategy to bring new innovations to a developing market to provide options for patients who currently do not have an adequate treatment for their uncontrolled drug-resistant hypertension.”
St. Jude Medical is conducting five renal denervation studies, including the EnligHTNment landmark trial. This study is the first large-scale trial that will examine the long-term effects of renal denervation to see if the therapy also reduces the risk of heart attack, stroke and heart failure requiring hospitalization, as well as cardiovascular death in patients with uncontrolled hypertension.
The next-generation EnligHTN system is currently being evaluated in the EnligHTN III study, an international, non-randomized clinical trial that will enroll up to 50 patients in Australia and New Zealand. This study expands on EnligHTN I trial research of the first generation EnligHTN system, which demonstrated that patients with drug-resistant hypertension had a safe, rapid and sustained drop in blood pressure. After thirty days, systolic blood pressure was reduced by an average of 28 mmHg that remained stable with a reduction of 27 mmHg one year after treatment.
For more information: www.sjm.com


 November 14, 2025
November 14, 2025 









