St. Jude Medical Receives CE Mark of Next-Generation Cardiac Device for Stroke Prevention
January 29, 2013 — St. Jude Medical Inc. announced European CE mark approval of its Amplatzer Amulet Left Atrial Appendage Occluder. The Amulet device is used to close the left atrial appendage (LAA) in patients diagnosed with non-valvular atrial fibrillation (AF). According to research, AF leads to an increased risk for stroke. Sealing off the LAA helps prevent the risk of blood clot formation and release, potentially reducing the risk of stroke. The next-generation percutaneous transcatheter device leverages the design and clinical success of the original Amplatzer Cardiac Plugwith additional features allowing treatment of a wider range of appendage anatomies. St. Jude Medical will be showcasing the new Amplatzer Amulet device during the 18th annual Boston AF Symposium at booth 319.
During AF, chaotic electrical signals in the heart’s upper chambers (atria) beat erratically and out of sync with the two lower chambers, resulting in poor blood flow. The LAA is a tube-shaped appendage connected to the left atrium of the heart that can potentially hold static blood during an episode of AF, increasing the likelihood of clot formation. Research shows that in AF patients, approximately 90 percent of all cardiac blood clots form in the LAA. If a clot forms in the LAA and is then released into the heart, it may enter blood circulation, travel to the brain, block a vessel and cause an ischemic stroke. The current standard of care to treat ischemic stroke in AF patients is blood-thinning medications, which comes with a lifetime of medical management and major, sometimes fatal, bleeding risks.
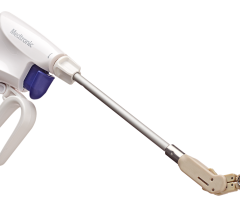
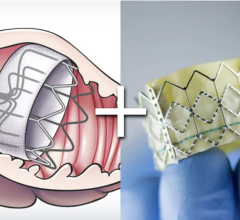
The enhanced Amplatzer Amulet design was driven by feedback from physicians who have been implanting the Amplatzer Cardiac Plug in Europe since 2008. Self-expanding and made of braided Nitinol mesh, consistent with the entire family of Amplatzer devices, the Amulet occluder works by blocking the LAA at its opening, which minimizes the opportunity for blood clots to form in the LAA or migrate into the bloodstream. This next-generation occlusion device is built with a longer lobe and waist than previous versions to allow for easier placement. The end screw is flush with the disc to create a smooth surface within the left atrium, and the larger disc diameter offers increased orifice coverage. The Amplatzer Amulet device is offered in eight sizes to accommodate varying anatomies. Additionally, the device is pre-loaded into the delivery catheter, which simplifies device preparation and ultimately streamlines the entire procedure for the physician.
“Feedback from implanting physicians who have used our first-generation product has been instrumental to improving an already successful device,” said Frank J. Callaghan, president of the Cardiovascular and Ablation Technologies Division. “We are pleased to offer a next-generation LAA occluder that addresses a wider range of patient anatomies, is easier to implant, and has the potential to further reduce the incidence of stroke in AF patients.”
According to the World Health Organization (WHO), an estimated 15 million strokes occur worldwide each year. In 2010, stroke cost the United States an estimated $53.9 billion in health care services, medications and missed days of work. Approximately 87 percent of all strokes are ischemic, which occur when blood clots block the blood vessels to the brain. Stroke is the third leading cause of death and the number one cause of long-term disability. AF is responsible for approximately 20 percent of ischemic strokes and about one-third of AF patients will have a stroke in their lifetime if not treated appropriately.
The Amplatzer Amulet Left Atrial Appendage Occluder is not yet approved for use in the United States.
For more information: www.sjm.com


 April 04, 2024
April 04, 2024