May 2, 2025 – New analysis from the EARLY TAVR trial showed patients between the age of 65 and 70 years old derived the ...
At the SCAI 2025 Scientific Sessions in Washington, DC, SCAI President James B. Hermiller, MD, MSCAI, recognized several ...
May 1, 2025 — A new expert opinion document jointly released by the Society for Cardiovascular Angiography and ...
May 1, 2025 – Analyses from the (Ergonomics in the Cardiac Catheterization Laboratory (ERGO-CATH) study show the ...
April 28, 2025 — iRhythm Technologies, Inc. recently announced results from a large real-world retrospective analysis ...
April 28, 2025 — The Society of Thoracic Surgeons (STS) has launched its latest surgical risk calculator designed for ...
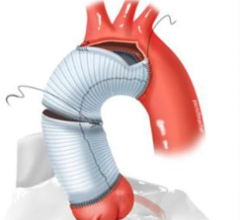
April 25, 2025 – Medtronic plc, a global leader in healthcare technology, recently received U.S. Food and Drug ...
April 25, 2025 — The annual Society for Cardiovascular Angiography & Interventions (SCAI) meeting, SCAI 2025 Scientific ...
April 25, 2025 – Findings from two late-breaking clinical trials demonstrate the safety and efficacy of left bundle ...
April 24, 2025 – The Heart Rhythm Society (HRS) has unveiled new research that underscores the critical role of ...
April 23, 2025 – The Heart Rhythm Society (HRS) has released a framework outlining criteria for establishing an Atrial ...
April 24, 2025 – The Heart Rhythm Society (HRS) and the American College of Cardiology (ACC) have released a scientific ...
April 22, 2025 — Research teams from Augusta University, Massachusetts General Hospital and The Ohio State University ...
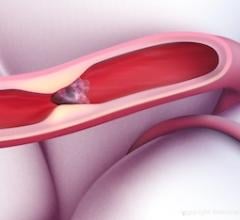
April 17, 2025 — Adults younger than 50 years of age had more than double the risk of having a stroke from migraine or ...
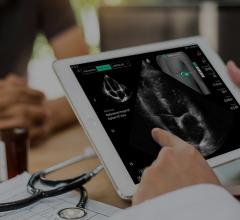
April 1, 2025 — UltraSight recently announced the presentation at the American College of Cardiology (ACC) Annual ...


 May 02, 2025
May 02, 2025