June 24, 2014 — Researchers have announced the results of a study that used speckle-tracking echocardiography (STE) to ...
Structural Heart
This structural heart channel includes news, videos, podcasts and other content related to diagnosis and treatment of structural heart disease. Topics covered include heart valve repair and replacement, transcatheter aortic valve replacement (TAVR), transcatheter mitral valve replacement (TMVR), transcatheter tricuspid valve replacement (TTVR), left atrial appendage (LAA) occlusion, heart failure interventional device therapies, and closing holes in the heart using, including occlusion of atrial septal defects (ASDs), ventricular septal defects (VSDs) and patent foramen ovales (PFOs).
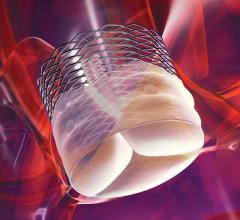
June 23, 2014 — Edwards Lifesciences Corp. announced that three-year clinical outcomes of its Edwards Intuity valve ...
June 20, 2014 — Less aggressive anticoagulation therapy, combined with low-dose aspirin, can be used safely in ...
As medical advancements continue to push the boundaries of what is possible in the field of structural heart ...

Interventional thought leaders at the American College of Cardiology (ACC) 2014 meeting shared their predictions about ...
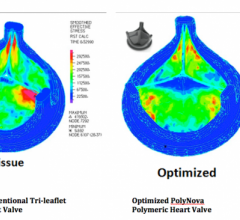
June 16, 2014 — PolyNova, a startup company that has grown out of an inter-institutional collaboration between the ...
June 16, 2014 — Boston Scientific Corp. has initiated the RESPOND post-market registry to assess real-world performance ...

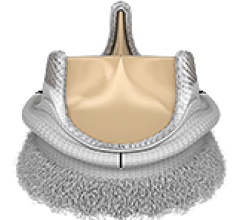
June 11, 2014 — Medtronic Inc. announced five-year follow-up data demonstrating the safety and performance of the ...
June 3, 2014 — Edwards Lifesciences Corp. received CE mark for the advanced Edwards Intuity Elite valve system. The next ...
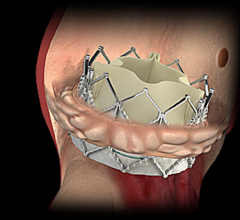
June 3, 2014 — JenaValve Technology Inc., a privately held, venture-backed developer of transcatheter aortic valve ...
May 30, 2014 — Direct Flow Medical has received investigational device exemption (IDE) approval from the U.S. Food and ...
May 30, 2014 — CardiAQ Valve Technologies announced it successfully implanted its second-generation transcatheter mitral ...
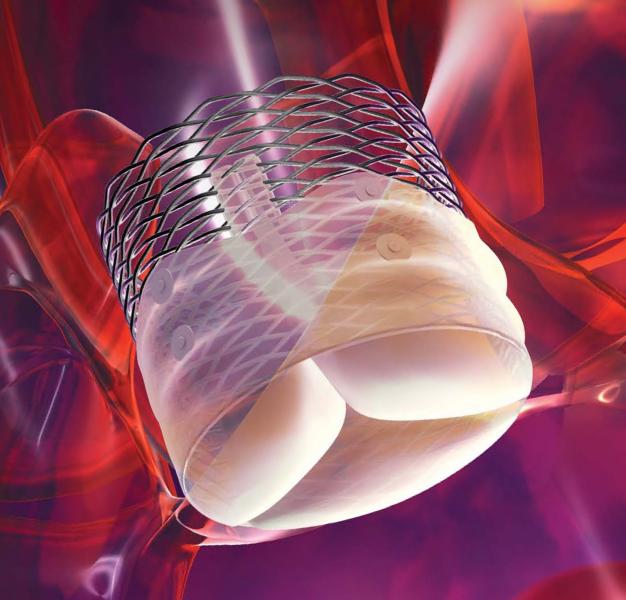
May 30, 2014 — Further validating its advanced transcatheter aortic valve implantation (TAVI) technology, the Boston ...
May 28, 2014 — Direct Flow Medical Inc. announced 12-month outcomes from the DISCOVER CE mark clinical trial. In the ...

 June 24, 2014
June 24, 2014