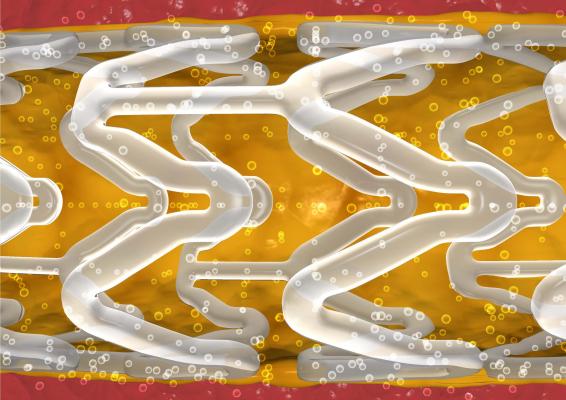
May 19, 2015 — Abbott announced that it has received CE Mark for the latest advancement of its Absorb stent system, called Absorb GT1. Absorb GT1 combines a fully dissolving stent with a next-generation delivery catheter to help doctors treat people with heart disease. Built upon three generations of delivery catheter innovations, Absorb GT1 refers to the GlideTrack catheter, Abbott’s advanced stent delivery system, which is designed to make it easier for doctors to access and treat diseased vessels in people with coronary artery disease (CAD). The GlideTrack catheter incorporates several design and technology changes that have the potential to improve deliverability and performance.
“Absorb GT1 will enable physicians to treat more people with coronary artery disease due to its improved delivery system,” said Christoph Kurt Naber, director of Contilia Heart and Vascular Centre, Essen, Germany. “Dissolving stents are the next logical step in the treatment of patients with coronary artery disease as these stents completely dissolve after opening up clogged arteries and restoring blood flow. With the prevalence of CAD around the world, this therapy has the potential to improve the health of many people.”
Last year, Abbott announced positive one-year clinical results from ABSORB II, the world’s first prospective, randomized, controlled trial comparing the safety and effectiveness of the fully dissolving Absorb heart device to Abbott’s metallic Xience family of drug-eluting stents. At one year, overall clinical outcomes for Absorb were comparable to Xience. The trial, conducted primarily in Europe, included 501 people with CAD. At EuroPCR, a scientific meeting for cardiologists held in Paris, Absorb data will be presented throughout the conference, which runs from May 19-21, 2015.
Absorb is currently available in more than 70 countries worldwide. The company recently completed its submission for regulatory approval of Absorb in Japan, and it plans to submit reports, including data from pivotal trials, for regulatory approvals in the United States and China in the coming months. Combined, these three countries represent more than 50 percent of the world’s heart stent procedures.
Currently, Absorb is an investigational device in the U.S. and is not approved for U.S. commercial use.
The device functions like a metallic stent by opening a blocked artery in the heart and restoring blood flow. However, unlike a metallic stent, which cages the vessel, Absorb is more flexible and dissolves over time, leaving behind a treated vessel free of a permanent implant with the potential to flex, pulse and dilate in response to various demands on the heart, based on people’s lifestyle and activities, such as exercise.
Abbott’s BVS delivers everolimus, an anti-proliferative drug used in Abbott’s Xience coronary stent systems.
For more information: www.abbott.com


 November 14, 2025
November 14, 2025 









