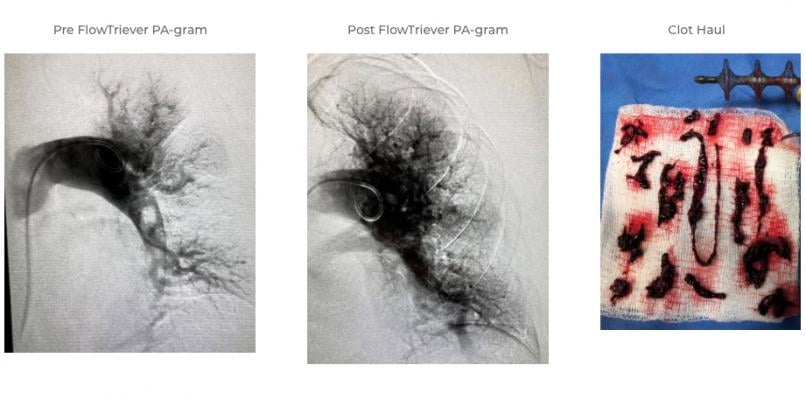
Patient case example of the FlowTriever thrombectomy device from Inari Medical Inc. in removing a pulmonary embolism and reporting blood flow and clot burden removed by the system.
October 23, 2020 — Positive results were reported on the first 230 patients enrolled in its FLASH study, a real world pulmonary embolism (PE) population, using the FlowTriever thrombectomy device from Inari Medical Inc. The FLASH Registry included high- and intermediate-risk patients, was obtained across 19 U.S. sites.
Results were presented virtually by National Principal Investigator Catalin Toma, M.D., director of interventional cardiology at UPMC Heart and Vascular Institute in Pittsburgh, Penn., 2020 Transcatheter Cardiovascular Therapeutics (TCT) Connect virtual symposium of the Cardiovascular Research Foundation (CRF).
FLASH is a 500-patient prospective, multicenter, single-arm registry evaluating real world patient outcomes after treatment of PE with FlowTriever. Of the first 230 patients enrolled, 98.7% (227/230) met the study’s primary endpoint of freedom from major adverse events at 48 hours. Secondary endpoints include impact on acute hemodynamics, procedural measures, 48h all-cause mortality, and longer-term patient outcomes. All secondary outcome measures analyzed showed statistically significant and clinically meaningful improvements from baseline.
“FLASH represents the largest prospective hemodynamic study of any PE treatment ever undertaken and is the first major all comers study of a purely mechanical thrombectomy approach to PE,” Toma said. “There were no deaths at 48-hour follow-up, no cardiac or pulmonary injuries, no procedure-related clinical deteriorations, and not a single instance of intracranial hemorrhage, a limitation of thrombolytic drugs. Hemodynamic parameters including pulmonary artery pressures and cardiac index improved significantly post procedure. These results were achieved in short, single session procedures. Additionally, by obviating the need for thrombolytic infusion, FlowTriever enabled patients to minimize stay in critically-needed ICU beds to a median duration of zero days following intervention.”
Immediate post-procedure hemodynamic improvements have not been demonstrated with thrombolytic-based approaches, which can take several hours to take effect. By contrast, after clot removal with FlowTriever, patient heart rates quickly improved by an average of 23 beats per minute. The majority (77%) of patients were tachycardic (>100 bpm) pre-procedure and only 25% were tachycardic immediately afterward. Similarly, the average pulmonary artery pressure dropped a remarkable 7 mmHg, with several patients normalizing immediately after clot removal.
“FLASH reflects Inari’s long-term commitment to venous thromboembolism (VTE) patients, and to producing a robust portfolio of clinical data to further the understanding and advance the treatment of this disease,” said Thomas Tu, M.D., chief medical officer of Inari Medical. “We remain committed to revolutionizing VTE treatment by continuing to build an armamentarium of purpose-built devices that remove large clot volume from large vessels while completely eliminating thrombolytics and their consequent cost, ICU stay, and bleeding complications.”
Inari Medical, Inc. is a commercial-stage medical device company focused on developing products to treat and transform the lives of patients suffering from venous diseases. Inari has developed two minimally-invasive, novel catheter-based mechanical thrombectomy devices that are designed to remove large clots from large vessels and eliminate the need for thrombolytic drugs. The company purpose-built its products for the specific characteristics of the venous system and the treatment of the two distinct manifestations of venous thromboembolism, or VTE: deep vein thrombosis and pulmonary embolism. The ClotTriever system is 510(k) cleared by the FDA for the treatment of deep vein thrombosis. The FlowTriever system is 510(k) cleared by the FDA for the treatment of pulmonary embolism.
Find additional TCT 2020 news, video and late-breaking studies


 January 05, 2026
January 05, 2026 









