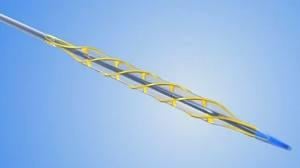
September 10, 2010 – Cath Labs are being encouraged this week by the U.S. Food and Drug Administration (FDA) to inspect their inventories for AngioSculpt EX Percutaneous Transluminal Coronary Angioplasty (PTCA) Scoring Balloon Catheters. Devices manufactured between Jan. 30 and Dec. 4, 2009 are part of a class 1 product recall due to safety concerns.
The FDA said the catheters may become separated during use, in which fragments of the catheter may become lodged in coronary arteries. This may result in serious injuries, including death. The recall only includes the EX catheters.
The recall was originally initiated on Dec. 4, 2009, but the FDA reissued the alert this week. The affected catheters include all part/REF numbers 2034-XXYY with lot numbers less than F09060003.
The AngioSculpt PTCA Scoring Balloon Catheter is used to dilate narrowed coronary arteries and to improve myocardial perfusion.
Class 1 recalls are the most serious type of recall and involve situations in which there is a reasonable probability that use of these products will cause serious adverse health consequences or death.
For more information: www.fda.gov


 June 13, 2024
June 13, 2024 









