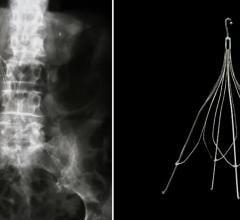
March 15, 2011 – Argon Medical Devices has entered into a definitive license agreement with Rex Medical, for exclusive global rights to market and distribute the Option Retrievable Inferior Vena Cava (IVC) Filter. The filter is an implantable device used in the prevention of pulmonary embolism (PE).
Argon will be exhibiting at the 36th Annual Society of Interventional Radiology (SIR) meeting, to be held in Chicago from March 27-30, 2011.
The nitinol, filter, with a low profile 5 French (6.5 French outer diameter) delivery system, is designed to be implanted into the inferior vena cava of patients to prevent recurrent PE. The filter is designed with symmetric flared struts to direct clot volume into the center of the vessel for maximum dissolution and preservation of blood flow allowing for capture of clinically significant clot and protection against PE. Designed as both a permanent or retrievable implant, the self-centering filter promotes optimal positioning and stability within the inferior vena cava. Its intuitive, easy-to-use design, makes deployment and retrieval safe and effective.
For more information: www.argonmedical.com


 July 27, 2021
July 27, 2021