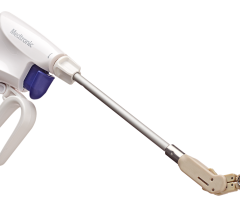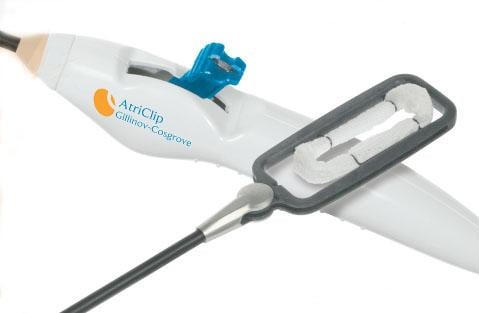
July 22, 2014 — AtriCure announced it has sold more than 34,000 AtriClip left atrial appendage (LAA) exclusion system devices worldwide.
The LAA is a muscular pouch attached to the heart’s left atrium. In patients with atrial fibrillation and other cardiac arrhythmias, blood can pool and form clots in the appendage, which may then leave the heart and cause strokes. One study concluded that more than 90 percent of detected blood clots in patients with atrial fibrillation are formed in the LAA.
For years, heart surgeons would remove the LAA with a cut-and-sew method during surgery, which required extra time on the heart-lung machine and posed a risk of hemorrhaging. In 2010, the AtriClip system was cleared for sale in the United States by the Food and Drug Administration (FDA) with the indication for occlusion of the LAA, under direct visualization, in conjunction with other open cardiac surgical procedures. The clearance was made after the EXCLUDE trial showed the LAA was closed successfully with the AtriClip device in 98.4 percent of patients, with no device-related mortality.
“The AtriClip device is quick to place and provides consistent occlusion of the appendage with minimal risk of hemorrhage," said J. Michael Smith, M.D., chief of cardiovascular surgery, director of robotics, co-director of transcatheter valve therapies and surgical research director of the Hatton Research Institute at TriHealth in Cincinnati.
In early 2014, AtriCure initiated the Stroke Feasibility study using the AtriClip system in a minimally invasive procedure on a beating heart. This study will evaluate the safely of the AtriClip when used for stroke prevention in patients with non-valvular atrial fibrillation who can’t take long-term anticoagulation medications. Complete exclusion of the LAA will be confirmed during the procedure using echo graphic imaging. The study will be conducted at seven hospitals in the United States, enrolling up to 30 patients.
“This study demonstrates our commitment to developing compelling solutions that improve patients’ lives and decrease the social and economic burden of atrial fibrillation,” said Mike Carrel, CEO of AtriCure. “The previous difficulty in obtaining complete appendage closure has largely been overcome with the use of the AtriClip system.”
For more information: www.atricure.com


 April 04, 2024
April 04, 2024