June 10, 2013 — C. R. Bard Inc. announced the enrollment of the first patient into the Lutonix Below the Knee (BTK) Clinical Trial at The Cardiac and Vascular Institute in Gainesville, Fla. The purpose of this pivotal global, multi-center randomized Investigational Device Exemption (IDE) trial is to compare the safety and effectiveness of the Lutonix 014 Drug Coated PTA Dilatation Catheter to a standard angioplasty balloon catheter for the treatment of critical limb ischemia.
Critical limb ischemia (CLI) is a severe blockage in the arteries of the legs or feet that significantly reduces blood flow. Limbs may develop painful sores, ulcers and/or gangrene (dead tissue) because they do not have enough oxygen. The pain may be severe, can last for hours, and typically occurs at night during rest times. If this condition is left untreated, patients may face the risk of amputation.
The Lutonix BTK clinical trial is expected to enroll several hundred patients at 55 sites worldwide. Patients will be randomized (2:1) for treatment with a Lutonix 014 DCB Catheter (study arm), or a standard non-coated angioplasty balloon (control arm). The Principal Investigators of the Lutonix BTK clinical trial are:
- Patrick Geraghty, associate professor of Surgery and Radiology at Washington University School of Medicine
- Jihad Mustapha, director of Endovascular Interventions, Metro Heart and Vascular, Metro Health Hospital, Wyoming, Mich., associate professor of Medicine, Michigan State University
- Professor Dr. Marianne Brodmann, associate professor and assistant medical director, Division of Angiology, Medical University Graz, Austria
Arthur Lee from The Cardiac and Vascular Institute stated, “This patient population faces significant challenges and poor clinical outcomes. Drug coated balloons potentially offer a new hope for more durable and long term clinical outcomes for patients facing CLI.”
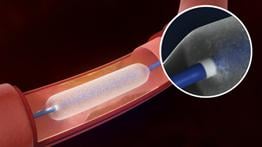
The Lutonix 014 DCB is similar to a standard angioplasty balloon, but is coated with an anti-proliferative drug (paclitaxel) designed to help keep arteries open and free from restenosis. The Lutonix 014 DCB catheter is not commercially available in the United States and is limited to investigational use under an investigational device exemption (IDE). The Lutonix 014 DCB catheter is commercially available in Europe.
The Lutonix BTK trial is one of several studies designed to produce long term clinical evidence of the Lutonix drug coated balloon in order to expand treatment options for peripheral arterial disease. Lutonix completed enrollment of 476 randomized patients last July for the Levant 2 IDE study for Femoral-Popliteal use and is actively recruiting patients for the Levant 2 Continued Access Safety Study.
For more information: www.crbard.com


 June 13, 2024
June 13, 2024 









