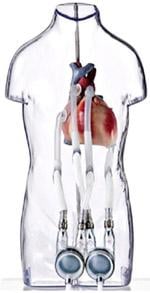
August 7, 2014 — The Berlin Heart Group announced they have completed enrollment in their post-approval study, the only condition of the humanitarian device exemption (HDE) approval that Berlin Heart received for the Excor pediatric ventricular assist device (VAD) on Dec. 16, 2011.
The Excor pediatric VAD is a mechanical cardiac support system for critically ill pediatric patients suffering from severe heart failure. The system is designed to support pediatric patients of all age groups, from newborns to teenagers, and is intended to bridge patients awaiting heart transplantation from days to several months, until a donor heart becomes available. The device, which is also approved for use in Europe and Canada, is the only ventricular assist device, designed specifically for the pediatric population, to be approved in the United States.
The purpose of the post-approval study for the Excor pediatric VAD was to evaluate whether safety and outcomes of the device in the commercial setting were comparable to the safety and outcomes of the device in the IDE study. The "all-comers" prospective study enrolled 39 subjects who were implanted with the device per the approved labeling. Of the 39 subjects enrolled, 34 have reached an endpoint to date. As soon as the remaining subjects reach an endpoint, the study data will be analyzed and presented to the U.S. Food and Drug Administration (FDA).
Bob Kroslowitz, president and CEO of Berlin Heart's North American operations, said, "We are extremely pleased that we have, with the continued support of our implanting sites, been able to satisfy the final requirement of the Excor pediatric approval in a very short period of time. As the Excor pediatric had extensive use prior to the HDE approval [available to all North American sites who requested the device under compassionate use regulations], we are confident that the results of the post-approval study will be consistent with the IDE study, and in some areas will trend more favorably."
For more information: www.berlinheart.com


 June 19, 2024
June 19, 2024 









