June 1, 2022 — Biofourmis, a Boston-based global leader in virtual care and digital medicine, announced that its Biofourmis Care solution has been selected by the American College of Cardiology (ACC) for its TRANSFORM3 study that will evaluate different intervention strategies for improving adherence to guideline-directed medication therapy (GDMT) for managing chronic cardiovascular conditions. The study will place an emphasis on underserved populations and those with a history of healthcare disparities.
TRANSFORM3 aims to solve the decades-long challenge in healthcare to promote and incentivize the adoption of guideline-directed care for cardiovascular conditions.
The study, “Evaluation of Implementation Strategies of Teaching, Technology, and Teams to Optimize Medical Therapy in Cardiovascular Disease (T3),” will include participants with: heart failure with preserved ejection fraction (HFpEF); heart failure with reduced ejection fraction (HFrEF); the heart rhythm condition atrial fibrillation; or cardiovascular risk among patients with type 2 diabetes.
Heart disease is the leading cause of death for men, women and people of most racial and ethnic groups in the United States. Access to care and improved care management can change those outcomes. For example, in the U.S., heart failure affects an estimated 6 million Americans, and poor heart failure management is the leading cause of hospitalizations and readmissions among older adults. However, only 25% of eligible patients receive GDMT and less than 1% are titrated to optimal doses.
“ACC’s selection of Biofourmis to take part in the TRANSFORM3 study signifies a growing recognition in healthcare that virtual care modalities need to be evaluated as viable, clinically prudent alternatives to more costly and time-consuming in-person clinic visits for management of patients with complex chronic conditions,” said Maulik Majmudar, MD, chief medical officer and co-founder of Biofourmis. “By the study’s conclusion, we are confident that the complete Biofourmis Care solution, including our licensed, multidisciplinary care teams focused on optimization of guideline-directed medical therapies, will demonstrate superior performance as compared with the current standards of care.”
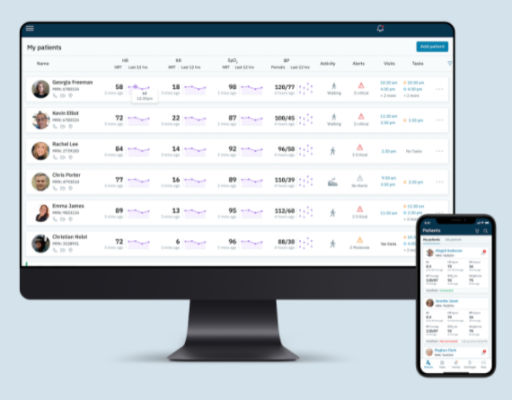
The Biofourmis Care platform—a first-of-its-kind, technology-enabled care management service that delivers high-quality remote care for patients with chronic conditions—includes connected devices, medication management algorithms, a patient-facing mobile app and a virtual multidisciplinary team. Each element of the platform will be evaluated against the standard of care for treating these conditions, which is in-person education and consultation between providers and patients. ACC selected Biofourmis to participate after an open request for proposals and review process.
The primary investigators of TRANSFORM3 are a team of physicians from Harvard Medical School practicing at Massachusetts General Hospital and Brigham and Women’s Hospital in Boston as well as St. Luke’s Health System in Kansas City.
“When patients have consistent access to healthcare services, they tend to have better therapies, adherence and outcomes; yet barriers to access often exist for many cardiovascular disease patients,” said Jim Januzzi, MD, FACC, principal investigator of TRANSFORM3, ACC Board of Trustees member, and director of the Dennis and Marilyn Barry Fellowship in Cardiology Research at Massachusetts General Hospital. “We hypothesize that care for patients with these conditions, including underserved populations, can be safely and effectively optimized with support outside the traditional ambulatory care encounters, by leveraging technology and dedicated care teams.”
Achieving GDMT: Overcoming Obstacles
To date, most patients and providers determine an optimal medication and dosage after several ambulatory office visits with several months in between. If the medication is ineffective, time is wasted while the patient’s condition deteriorates. Or the drug may cause intolerable side effects leading to patient nonadherence to the therapy, which may also worsen the condition.
To learn how providers can avoid these common scenarios, patients will be assigned at random to either the Teach, Technology or Teams study arms. The Teach cohort will be provided with educational materials on the most up-to-date guideline recommendations, while the Technology cohort will have access to the Biofourmis Care solution—including connected devices and medication optimization algorithms—but will be managed by local providers. The Team cohort will leverage the Biofourmis Care solution as well, but will also be remotely supported by frontline Biofourmis health navigators who coordinate with a multidisciplinary team, including nurses, physicians or other specialty care practitioners. The health navigator reviews and responds to notifications from the Biofourmis Care solution and serves as the first resource for patients’ clinical concerns or technical troubleshooting. The health navigator also triages and escalates concerns to the appropriate clinician, who then provides higher level clinical expertise as needed. Critical notifications are always escalated and sent directly to the patient’s physician, and the electronic health record (EHR) is updated accordingly. Part of the decision support of the software includes automated medication management to augment and assist care teams in remotely adjusting medications for optimal personalized therapy and detecting patterns of clinical deterioration for earlier interventions.
Investigators will also collect information about the patient experience, including their cardiovascular disease knowledge, preferences for treatment and barriers to GDMT adherence such as concerns about cost or safety and efficacy.
“TRANSFORM3 will provide real-world data on how cardiologists and other clinicians can more effectively and efficiently manage chronic cardiovascular conditions in underserved populations,” said Megan Welch, MD, TRANSFORM3 investigator team member and cardiovascular disease fellow at Massachusetts General Hospital. “Through technology-enabled approaches, we are hopeful that providers will have timely, meaningful awareness of their patients’ health status and adherence to guideline-recommended therapies. Ultimately, what we learn from TRANSFORM3 could lead to accelerated adoption of effective, evidence-based care plans that optimize outcomes and help patients lead longer, healthier lives.”
For more information: www.biofourmis.com


 July 31, 2024
July 31, 2024 









