September 9, 2016 — Six-month preclinical data from trials of a Xeltis bioabsorbable aortic conduit were presented at the 2016 scientific meeting of the International Society for Applied Cardiovascular Biology (ISACB). The data was presented by Prof. Frederick Schoen, M.D., Ph.D., senior pathologist and executive vice chairman, Department of Pathology at Brigham and Women’s Hospital, and professor of pathology and health sciences and technology, Harvard Medical School.
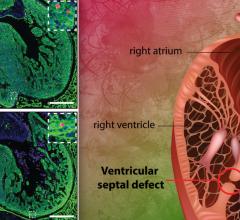
The data showed that a Xeltis bioabsorbable aortic conduit enabled endogenous tissue restoration (ETR) in the systemic circulation, the part of the cardiovascular system flowing via the aorta throughout the body. Systemic circulation is characterized by higher blood pressure than pulmonary circulation, a loop through the heart and lungs. Xeltis bioabsorbable cardiovascular technology has already been proven to effectively enable ETR in pulmonary circulation.
Watch a VIDEO animation of how the Xeltis valve is supposed to work
Clinical data presented at the American Association for Thoracic Surgery in May 2016 showed anatomical and functional stability of Xeltis devices in a feasibility study of five pediatric patients and no adverse events at one-year follow-up. Indeed, two-year follow-up data are expected to be ready soon and presented at a major scientific meeting.
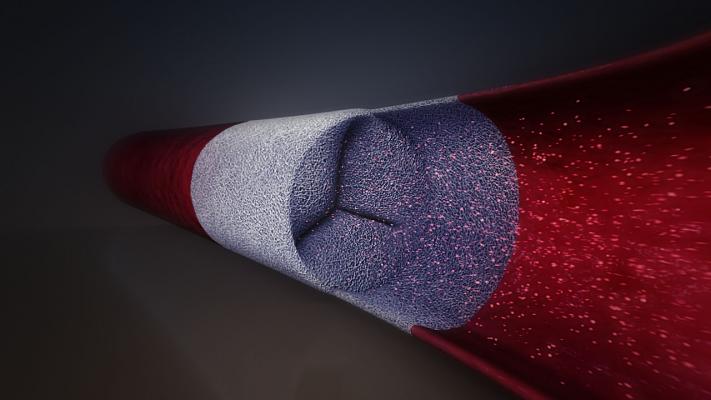
According to the company, Xeltis technology is the first-ever cardiovascular regenerative medicine platform based solely on a bioabsorble device. By pervading the porous Xeltis matrix, new tissue forms around and inside it and rebuilds a new heart valve or blood vessel. Xeltis devices are designed to absorb over time, leaving patients with a new, healthy, functioning heart valve or blood vessel. Current valves are plagued with complications: the potential for rejection, calcification and chronic infection.
Preclinical data was initially presented by Schoen at the 10th World Biomaterials Congress in June 2016 validating the potential of Xeltis devices to “guide the restoration of a patient’s natural tissue into a functional living vascular replacement.”
The Xeltis platform is the first to apply the principles of supramolecular chemistry and the properties of bioabsorbable polymers to enable cardiovascular regenerative medicine via implanted medical devices. The ETR process enabled by Xeltis devices does not require any in vitro tissue engineering, stem cells or other biological agents.
The platform is protected by a portfolio of 20+ international patent families, including Xeltis’ supramolecular polymer platform and its electrospinning methodology for manufacturing the devices. Electrospinning is a fiber production method that uses electric force to draw solid, charged threads from polymer solutions or polymer melts up to fiber diameters a fraction of the diameter of a hair.
Jean-Marie Lehn, Ph.D., co-winner of 1987 Nobel Prize in Chemistry, said, “Supramolecular chemistry enables Xeltis technology by providing unique biochemical and biomechanical properties, delivering solutions to issues faced by traditional materials over the course of decades.”
Schoen said, “I am impressed by the Xeltis preclinical results to date that have advanced our understanding of host/biomaterial interactions and show potential for an innovative approach that could improve the care of patients with cardiovascular disease.”
Martin B. Leon, M.D., director, Center for Interventional Vascular Therapy, Columbia University Medical Center/New York-Presbyterian Hospital, said, “I am excited by the potential of the Xeltis technology for the replacement of heart valves, bringing significant benefits not only to the procedure and valve designs but also to clinical outcomes for patients.”
Lehn, Schoen and Leon are scientific advisors to Xeltis. The Xeltis technology is investigational and not available for sale.
For more information: www.xeltis.com


 March 31, 2025
March 31, 2025