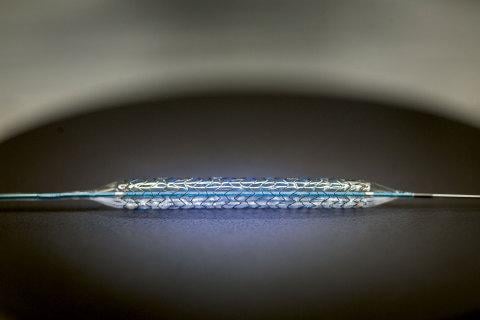
December 27, 2012 — Biotronik announced that the first implant has taken place in the BIOHELIX-I trial in the United States. The BIOHELIX–I trial is set to evaluate the safety and performance of the Pro-Kinetic Energy coronary bare metal stent. The first implant was successfully performed by H. Barrett Cheek of Carolina Cardiology Cornerstone at High Point Regional Hospital in High Point, N.C.
The BIOHELIX-I trial is a prospective, nonrandomized, multicenter, investigational device exemption (IDE) study that will utilize Biotronik’s Pro-Kinetic Energy coronary stent system in patients with symptomatic, ischemic heart disease. More than 300 patients will be enrolled in the study, which is registered on www.clinicaltrials.gov.
Saurabh Gupta, M.D., director of the cardiac catheterization laboratory at Oregon Health & Science University in Portland, Ore., is the national principal investigator of the BIOHELIX-I study. “The thin cobalt chromium struts of the Pro-Kinetic Energy allow for greater flexibility and a reduced crossing profile, which translates into a highly deliverable stent,” Gupta said.
He added, “The clinical safety and performance of the Pro-Kinetic Energy stent has been demonstrated in recent results of the ENERGY registry, presented at the TCT 2012 conference. The BIOHELIX-I study will provide additional insight and experience into the clinical application of the Pro-Kinetic Energy stent for patients with ischemic heart disease.”
The Pro-Kinetic Energy is a thin strut (60?m/0.0024 inches) cobalt chromium, bare metal stent, completely sealed with a thin layer of amorphous silicon carbide called PROBIO. This passive coating is designed to reduce the amount of ions released from the stent, potentially reducing adverse reactions post-implantation.
The ENERGY registry was an all-comers registry of more than 1,000 patients with a complex population of 39 percent B2/C lesions and 46 percent acute coronary syndrome patients. At 12 months, Pro-Kinetic Energy showed a low 8.8 percent rate of major adverse cardiac events (MACE), including a 3.4 percent rate of target lesion revascularization (TLR). A subgroup analysis showed similarly low 12-month MACE rates in acute coronary syndrome (ACS) patients, elderly patients, and patients with small vessels or diabetes.
“Biotronik is very pleased to offer this state-of-the-art coronary stent technology to U.S. patients enrolled in the BIOHELIX study,” said Mark Johnson, program director for Biotronik’s U.S. vascular intervention business. “The Pro-Kinetic Energy provides both patients and clinicians with an optimal choice for their coronary vascular therapy needs.”
For more information: www.biotronik.com


 January 05, 2026
January 05, 2026 









