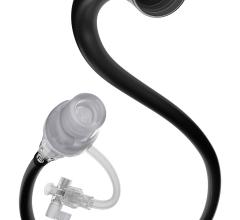
February 4, 2013 — Biotronik recently launched its 3Flow Aspiration Catheter in Europe. The catheter is specifically designed to facilitate quick and accurate thrombus aspiration, complementing Biotronik’s already broad coronary vascular intervention (CVI) portfolio.
Thrombus aspiration is applicable in a larger majority of patients with myocardial infarction with ST-segment elevation (STEMI). Acute ST-segment elevation indicates that a relatively large amount of heart muscle damage is occurring because thrombus occludes an epicardial artery. Thrombus aspiration significantly improves the clinical outcome in STEMI patients and has received a IIa recommendation in the most recent ESC guidelines (European Society of Cardiology).
The 3Flow Catheter’s features differentiate it from other catheters on the market: With the supplied 60ml locking syringe and large inner lumen, the 3Flow Catheter allows for the extraction of larger thrombotic particles — as well as for a longer aspiration time. Its hydrophilic distal coating and smooth tip design furthermore ensure that excellent deliverability and optimal kink resistance are provided by the proximal high performance thermoplastic polymer shaft.
“The large syringe doubles the aspiration time compared to standard 30 mL syringes, which avoids switching syringes in many cases and allows to better focus on the accuracy of the procedure,” says Dr. Henrik Schneider from the University Hospital in Rostock, Germany. “At the same time, the large inner lumen catheter allows for aspiration of larger thrombotic particles and causes less blockages.”
“For STEMI patients, 3Flow is an ideal extension to our stent and balloon portfolio. In this very critical patient segment, a proper lesion preparation is as always key to successful stenting”, says Alain Aimonetti, vice president sales and business development, Biotronik Vascular Intervention.


 January 19, 2026
January 19, 2026