October 26, 2012 — Biotronik began its European market release of BioMonitor, an implantable cardiac device designed for accurate and reliable monitoring and management of patients with atrial fibrillation (AF) or unexplained syncope.
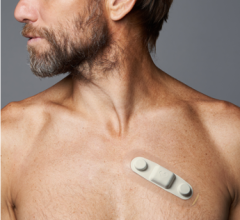
BioMonitor is a subcutaneous implantable leadless cardiac monitor for the long-term continuous remote monitoring of patients with arrhythmias such as AF, bradycardia, sudden rate drop, asystole and tachycardia.
As sensitivity and specificity are essential in the detection of arrhythmias such as AF, Biotronik has developed ClearSense Technology with a unique three-vector signal detection that produces highly precise and reliable arrhythmia monitoring.
The ClearSense Technology records three ECG channels converting them to one high quality ECG input signal to clearly distinguish on a beat-to-beat basis between a genuine signal and other artifacts such as myopotentials due to body muscle contractions. This innovative technology allows for optimal arrhythmia detection independent of the device’s implant orientation within the body and provides a longevity of 6.4 years.
BioMonitor is equipped with Biotronik’s home monitoring system, which provides daily remote data transfer without patient interaction — leading to high patient compliance. Its traffic light system streamlines monitoring by highlighting the most relevant information and providing accurate data for physicians to monitor and manage their patients effectively.
“Only long-term continuous monitoring with reliable arrhythmia detection offers the type of vital information necessary for physicians to make the right therapy decisions when managing patients with AF or unexplained syncope,” commented Professor Gerhard Hindricks, director of the department of electrophysiology at the Leipzig University Heart Center in Germany.
Hindricks together with professor Wilhelm Haverkamp (deputy of the department of cardiology at Charité Campus Virchow, Humboldt University Berlin) and professor Dietmar Bänsch (director of cardiology department and rhythmology at the Rostock University Hospital) are the world’s first physicians to implant the BioMonitor and take advantage of this new approach for monitoring AF in their patients.
“Effective management of AF and unexplained syncope starts with effective monitoring,” said Haverkamp. “BioMonitor, with its ClearSense technology, and Biotronik Home Monitoring offer the optimal combination of reliability and efficiency.”
The BioMonitor device marks a new product category in Biotronik’s device portfolio of pacemakers, implantable cardioverter-defibrillators and cardiac resynchronization therapy systems.
“BioMonitor supports physicians in every step of arrhythmia management, from diagnosis via monitoring to individualized therapy offering high quality solutions that benefit both physicians and patients,” explained Bänsch.
Sites involved in the first implantations of the BioMonitor:
- Germany
Prof. Gerhard Hindricks, Leipzig University Heart Center
Prof. Wilhelm Haverkamp, Charité Campus Virchow,Humboldt University Berlin
Prof. Dietmar Bänsch, RostockUniversityHospital
Prof. Dietrich Andresen, Vivantes Klinikum Am Urban/Im Friedrichshain, Berlin
Dr. Dieter Bimmel, St-Marien-Hospital, Bonn
- Italy
Dr. Gaetano Senatore, Ospedale Civile di Cirie
- Spain
Dr. Jesús Rodríguez García, University Hospital Doce de Octubre, Madrid
- Switzerland
Dr. David Hürlimann, University Hospital of Zurich
Dr. Jan Steffel, University Hospital of Zurich
- France
Dr. Arnaud Lazarus, Clinique Ambroise Paré, Paris
- Denmark
Dr. Jens Brock Johansen, Odense University Hospital
For more information: www.biotronik.com


 March 31, 2025
March 31, 2025