March 2, 2017 — BioVentrix Inc. announced that InEk, the German Institute for the Hospital Remuneration System, has awarded NUB Status 1 for the Revivent TC TransCatheter Ventricular Enhancement System for heart failure (HF).
The NUB process supports introduction of new and innovative medical devices in Germany. It allows participating hospitals to receive full reimbursement and a supplemental payment for new technologies in the German reimbursement system. NUB Status 1 is the highest priority designation available, and was only assigned to a small minority of product submissions for 2017.
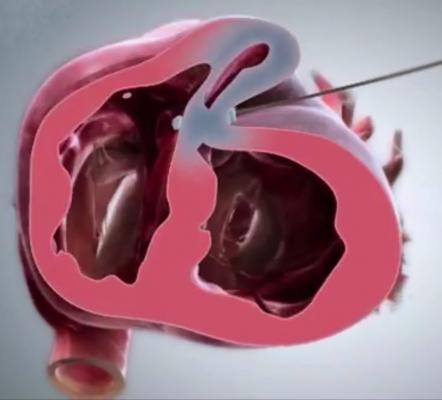
BioVentrix recently received CE mark certification for its closed-chest Revivent TC TransCatheter Ventricular Enhancement System for plication of scar tissue in post-myocardial infarction (MI), ischemic cardiomyopathy patients. Placement of the Revivent TC System via the LIVE procedure obviates the need for more invasive surgery. Instead, small titanium anchors are placed along the outer surface of the heart and along one of the interior walls via a catheter-based approach. The anchors are then pulled towards one another, effectively excluding the scarred and non-functioning heart wall. Ventricular volume is immediately reduced as a result of the exclusion, by as much as 30-40 percent.
For more information: www.bioventrix.com


 November 14, 2025
November 14, 2025 









