
The Reliance 4-Front lead is not currently available for sale in the United States.
April 28, 2014 — Boston Scientific Corp. has conducted the first implant in the clinical trial of the next-generation Acuity X4 left ventricular (LV) pacing leads and Reliance 4-Front implantable converter defibrillators (ICD) leads. The clinical trial is designed to establish the safety and effectiveness of both lead families and is intended to support U.S. Food and Drug Administration (FDA) approval of these devices.
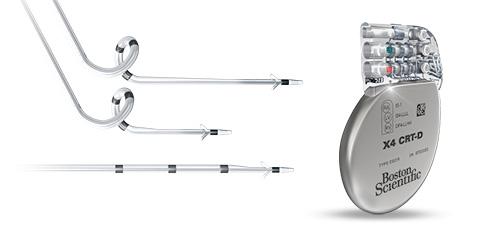
Acuity X4 LV leads are quadripolar leads engineered to maximize effectiveness and minimize unnecessary patient interventions after implant. The unique 3-D spiral design is intended to minimize floating electrodes, resulting in better electrical performance. In addition, dual-fixation zones and the 2.6 French crossing profile are designed to optimize lead stability and reduce the risk of dislodgement. When an Acuity X4 lead is connected to a Boston Scientific X4 CRT-D, the resulting 17 pacing vector options are designed to improve physicians' ability to manage unexpected complications electronically through programming rather than an invasive lead revision.
The Reliance 4-Front ICD lead is designed for reliability and performance over the long term. The lead maintains the same design principles as the Endotak Reliance lead, yet offers improved handling and maneuverability. Additionally, the Gore coating securely adhered to the defibrillation coils is intended to prevent tissue in-growth and enable both a streamlined implant experience and easier extraction in case of infection.
The first patient enrollments occurred at Wheeling Hospital in Wheeling, W. Va., with Maninder Bedi, M.D., and at Central Baptist Hospital in Lexington, Ky., with Aaron Hesselson, M.D. "The Acuity X4 portfolio allowed me to choose a lead that matched the patient's anatomy," said Hesselson. Likewise, Bedi noted the advantages of the Acuity X4 portfolio for his patient. "This patient may have otherwise had to go for an epicardial lead due to the lack of placement options, but the X4 lead got into several branches of a narrow anatomy that other leads may not have been able to access," said Bedi.
The Navigate X4 trial is a prospective, non-randomized, multicenter, global clinical study designed to support FDA approval. The two principal investigators are Suneet Mittal, M.D., director, electrophysiology, Valley Health System, and Martin C. Burke, D.O., professor of medicine and director, Heart Rhythm Center, University of Chicago Medical Center. The trial is expected to enroll between 1,542 and 2,290 patients at up to 125 centers in the United States, Canada and Israel. The Acuity X4 and Reliance 4-Front leads will be connected to commercially available Boston Scientific X4 CRT-D devices.
The Acuity X4 LV pacing leads and Reliance 4-Front defibrillator leads are CE marked. In the United States, they are investigational devices and not available for sale.
For more information: www.bostonscientific.com


 May 02, 2025
May 02, 2025 









