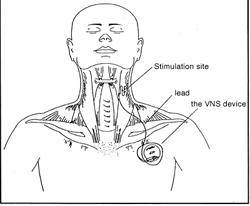
October 3, 2011 – Boston Scientific has enrolled the first patients in its NECTAR-HF (NEural Cardiac TherApy foR Heart Failure) clinical trial. NECTAR-HF is a prospective, randomized, international clinical feasibility study designed to assess preliminary safety and efficacy of chronic vagal nerve stimulation in heart failure patients. The study will evaluate 96 patients with vagal nerve stimulator implants at multiple centers in Europe.
"Stimulating the vagus nerve in the cervical region is commonly used to treat epilepsy and depression; however, promising pre-clinical data show that this therapy may also help a large population of heart failure patients who are currently not candidates for heart failure device therapy," said principal investigator and Steering Committee Chairman Faiez Zannad, M.D., Ph.D., professor of therapeutics and cardiology and director of clinical investigation center at INSERM in Nancy, France.
The first implants in the NECTAR-HF trial occurred in Barcelona, Spain, at Hospital Clinic by Jordi Rumia, M.D., (Maria Angeles Castel Lavilla, M.D., Ph.D, as principal investigator) and in Nancy, France at CHU Nancy-Brabois by Mazen Elfarra, M.D., (Zannad as principal investigator).
"NECTAR-HF will study whether vagal nerve stimulation can restore autonomic balance and therefore improve heart function, increase exercise capacity and inhibit the progression of heart failure," said co-principal investigator and Steering Committee member Josep Brugada, M.D., Ph.D., professor of medicine and medical director of Hospital Clinic in Barcelona.
"Our goal with vagal nerve stimulation therapy is to offer another treatment option for heart failure patients," said Kenneth Stein, M.D., chief medical officer, cardiac rhythm management for Boston Scientific. "This novel technology leverages Boston Scientific's capabilities in cardiac rhythm management device therapy, lead technologies and neuromodulation. This treatment capability is one of our Priority Growth Initiatives, and illustrates our strategy to offer new therapies in high-potential markets that are designed to improve the quality of life for patients."
Congestive heart failure affects nearly 23 million people worldwide, with approximately 2 million new patients diagnosed annually. Despite substantial advances over the past two decades in pharmacological and device therapy, heart failure remains one of the leading causes of morbidity and mortality in the United States and most European countries.
For more information: www.bostonscientific.com


 January 05, 2026
January 05, 2026 









