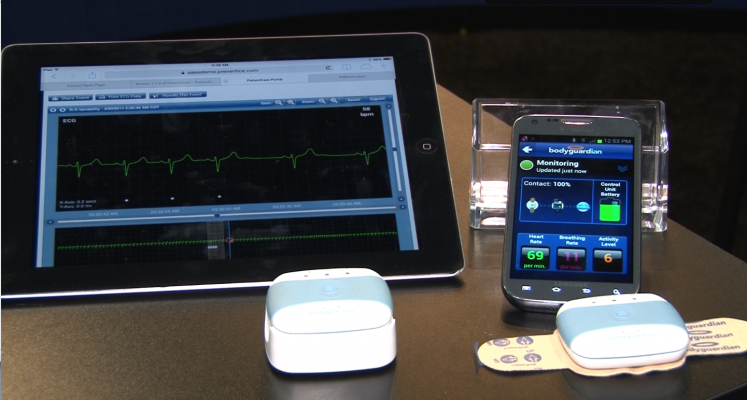
BodyGuardian product family. Image courtesy of Preventice Solutions.
August 11, 2015 — Boston Scientific has become a significant shareholder and will become the exclusive worldwide sales and marketing representative for Preventice Solutions for cardiology-related diagnostic and monitoring offerings.
Through this alliance, Boston Scientific will expand its commercial presence into the $1 billion cardiac diagnostics and monitoring market by gaining access to a wireless monitoring portfolio linked to an integrated remote monitoring platform.
Preventice Solutions, a privately held company, develops mobile health solutions and services and offers a full portfolio of wearable cardiac monitors, including Holter monitors, cardiac event monitors and mobile cardiac telemetry. These technologies are complemented by monitoring services including data transmission, surveillance, reporting, interpretation and integration into a healthcare provider's workflow.
"As our healthcare environment continues to evolve, healthcare practitioners, administrators and payors are looking for solutions that identify relevant clinical insights from large volumes of patient data and integrate those insights to improve clinical decision-making," said Joe Fitzgerald, executive vice president and president, rhythm management, Boston Scientific.
Cardiac monitoring is a critical component required for the diagnosis of many arrhythmias that require clinical intervention. Preventice Solutions' portfolio includes the PatientCare Platform and the BodyGuardian family of products.
For more information: www.bostonscientific.com


 November 14, 2025
November 14, 2025 









