April 8, 2014 — Cardinal Health announced an agreement to acquire privately held AccessClosure Inc. for $320 million. Subject to customary closing conditions and regulatory clearances, the all-cash transaction is expected to close by early June 2014.
Cardinal Health expects the transaction to have minimal impact on non-GAAP earnings in fiscal year 2014 and be slightly accretive to non-GAAP earnings in fiscal 2015.
“We are excited about this opportunity because it provides a scalable platform — with an outstanding product, strong customer base, cost-effective service model, and seasoned management team. Our goal is to become the partner of choice for solutions that improve patient care while reducing the cost and complexity of procedures for hospital systems,” said Don Casey, CEO of Cardinal Health’s Medical Segment. “Last year we launched our orthopedic trauma solutions to great response, and this acquisition will broaden our offering in another physician preference category. We look forward to further expansion.”
Gregory Casciaro, president and CEO of AccessClosure, said, “We are extremely pleased to join a healthcare solutions company like Cardinal Health which has existing scale and a structure to grow this business more rapidly and meet the needs of our current and future customers. With Cardinal Health’s resources and customer relationships, we can now more readily answer market demand for our innovative solutions that improve both the doctor and patient experience.”
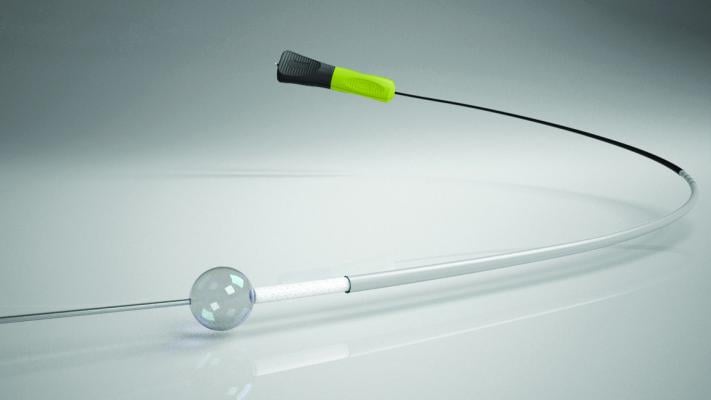
AccessClosure’s annual sales are more than $80 million, driven by a direct sales force. The company’s Mynx family of patient-friendly vascular closure devices helps physicians seal the femoral artery using a unique, secure sealant, which dissolves within 30 days, leaving nothing behind but a healed artery. Mynx allows patients to safely ambulate more quickly, which in turn, means patients can return home sooner.
Shaden Marzouk, vice president clinical affairs for Cardinal Health noted, “This acquisition will create a platform for an interventional suite offering that is differentiated in the marketplace. Cardinal Health offers customers and their patients high quality, value-priced solutions that include other services, resources, and tools for service line optimization. We know that these innovative, patient-centered solutions are extremely important in today’s evolving healthcare landscape.”
For more information: www.accessclosure.com


 October 27, 2021
October 27, 2021 







