August 18, 2022 — Six digital healthcare start-ups from five countries have officially become the first cohort of the Edison Accelerator in Canada – a program orchestrated by GE Healthcare.
The selected companies all focus on applying Artificial Intelligence (AI) to augment medical imaging with the potential to transform how healthcare is delivered. AI is poised to increase productivity and the efficiency of care delivery and allow healthcare systems to provide better care to more people. AI can help improve healthcare practitioners' experience, enabling them to spend more time in direct patient care and reducing costs.
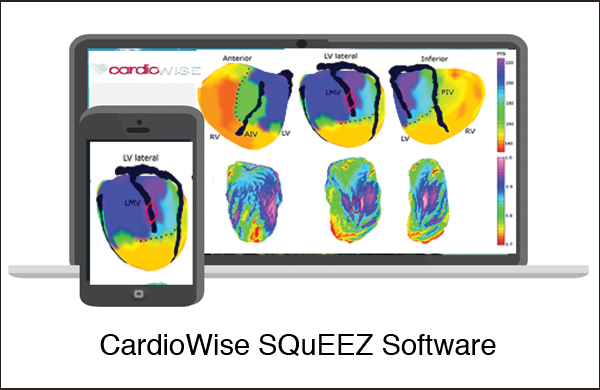
CardioWise SQuEEZ Software is an image analysis software application for cardiac Computed Tomography (CT) studies to assist cardiologists and radiologists in assessing heart function. The software calculates a Stretch Quantifier for Endocardial Engraved Zones (SQuEEZ) value to highlight and color code abnormalities in the wall motions of the heart. The cloud-based Software as a Service analysis is an intuitive, quantitative method to classify LV contractile function that has the potential to provide significant improvement in clinical decision-making relative to the inconsistent, qualitative tests used today.
Over the next three months, the chosen companies will integrate their solution within the Edison Digital Health Platform, which takes a vendor-agnostic approach to developing and deploying Artificial Intelligence solutions integrated within their clinical and operational workflow platforms. Each company has a customized program plan with the aim to accelerate integration and commercialization. The program culminates with an Innovation Showcase, during which all participants will present to a network of investors, potential business partners and customers who could help take their companies to the next level.
CardioWise has received the applicable regulatory clearances, including the FDA 510(k) and ISO 13485, necessary for distribution in the USA and the European Union. After the successful integration and term sheet agreements with GE, CardioWise will have the opportunity to distribute its products through the GE Healthcare Edison Marketplace. This marketplace is GE Healthcare’s online store that allows customers to find and buy the CardioWise applications that have been successfully integrated directly into the GE clinical and operational workflows to help healthcare providers improve patient care.
Jack Coats, CEO of CardioWise said, “We are very pleased to have been chosen to participate in the first cohort of the Edison Accelerator in Canada by GE Healthcare and Nex Cubed to accelerate CardioWise SQuEEZ AI cardiac functional analysis that provides a quantitative evaluation of heart function. CardioWise SQuEEZ analysis can provide caregivers with an easy-to-read, quantitative 4D color model that allows physicians to make better, more informed decisions about their patient’s care.”
For more information: https://www.cardiowiseinc.com/


 February 02, 2026
February 02, 2026 









