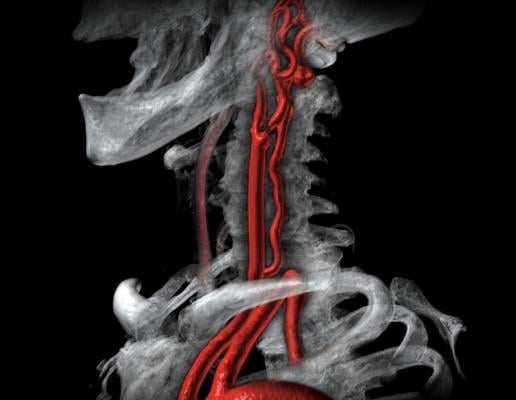
A 3-D CT reconstructed image showing a narrow lesion at the carotid bifurcation. ACST-2 was the largest trial to compare the long-term effect of carotid artery stenting (CAS) and carotid endarterectomy (CEA) on stroke in asymptomatic patients with a severely narrowed carotid artery that had not yet caused a stroke. Image by Canon
September 7, 2021 — Carotid artery surgery and stenting have comparable long-term effects on fatal or disabling stroke in asymptomatic patients with severe carotid artery stenosis. The ACST-2 trial late-breaking Hot Line session at the European Cardiology Society (ESC) Congress 2021 was published simultaneously in The Lancet.[1]
Patients with severe carotid artery stenosis are at elevated risk of stroke and both carotid artery stenting (CAS) and carotid artery surgery, also called carotid endarterectomy (CEA), can restore patency and reduce the long-term risk of stroke. Nationwide registry data from Germany have shown among asymptomatic patients, CAS and CEA are both associated with an approximately 1% risk of disabling stroke or death.[2] Comparative data are lacking on the long-term protective effects of the two procedures.
“We have shown that, for patients with a severely narrowed carotid artery, stenting and surgery have similar effects on the chances of having a disabling or fatal stroke. The risk from each procedure is about 1%. After that, however, the annual risk over the next five or more years is halved, from 1% down to 0.5% per year,” explained principal investigator Professor Alison Halliday of the University of Oxford, U.K.
ACST-2 was the largest trial to compare the long-term effect of CAS versus CEA on stroke in asymptomatic patients with a severely narrowed carotid artery that had not yet caused a stroke. The trial enrolled patients with severe carotid artery narrowing (60% or more reduction in diameter on ultrasound) found by chance, but with no recent stroke or other neurological symptoms. Participants were thought by their doctor to need CAS or CEA but both doctor and patient were substantially uncertain about which procedure was preferable.
A total of 3,625 patients were enrolled from 130 centres in 33 countries. Participants were randomly allocated 1:1 to CAS or CEA and followed up for an average of five years. The main outcomes were: 1) procedural risks (morbidity and mortality within one month after the procedure); and, most importantly, 2) non-procedural stroke, subdivided by severity.
Regarding procedural risks, 1% of patients in both groups had a disabling stroke or died within 30 days (15 allocated to CAS and 18 to CEA) and 2% had a non-disabling procedural stroke (48 allocated to CAS and 29 to CEA).
The main outcome was five-year non-procedural stroke; fatal or disabling stroke occurred in 2.5% of patients in each group, for a rate ratio (RR) of CAS versus CEA of 0.98 (95% confidence interval [CI] 0.64–1.48; p=0.91), and any non-procedural stroke occurred in 5.3% of the CAS group versus 4.5% of the CEA group (RR 1.16; 95% CI 0.86–1.57; p=0.33). A meta-analysis of this and all other major trials of CAS versus CEA yielded a similarly non-significant result for any stroke (RR 1.11; 95% CI 0.91–1.32; p=0.21).
ACST-2 is currently funded from the core support from the Medical Research Council (MRC), the British Heart Foundation, and Cancer Research UK for the University of Oxford’s NDPH. The trial had grants from the BUPA Foundation (BUPAF/33a/05) and National Institute for Health Research Health Technology Assessment program (HTA06/301/233) until 2013.
Find more ESC Hot Line late-breaking study news
References:


 January 05, 2026
January 05, 2026 









