February 16, 2012 — Few hospitals have as much experience with the Berlin Heart pediatric ventricular assist device (VAD) as DMC Children's Hospital of Michigan. This is why the U.S. Food and Drug Administration (FDA) was eager to hear testimony from Henry L. Walters III, M.D., the hospital’s chief of cardiovascular surgery. He provided testimony about the device at a recent public hearing. After reviewing the clinical trial data and hearing testimony from Walters and others, the device was approved.
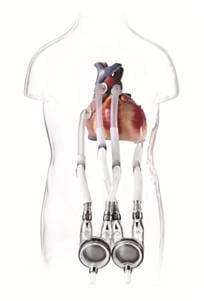
The Berlin Heart is the first VAD made specifically for pediatric patients. VADs are common in adults, but, until recently, were not manufactured in pediatric sizes.
Ventricular assist devices are used when a patient needs a heart transplant, but can't wait for a donor heart to become available. The Berlin Heart assists the failing heart until an appropriate donor heart can be found.
"A few times a year, we are faced with a situation where a child simply can't wait for a heart transplant," Walters said. "In these cases, the Berlin Heart works very well and the children generally stabilize and start to thrive and improve while they wait for a donor heart."
Before the device was approved by the Walters and his colleagues used the Berlin Heart under the FDA's "compassionate use" regulations.
Since 2005, Walters and his colleagues have inserted 14 Berlin Hearts. Of the 14 patients, 10 survived to cardiac transplantation and nine are thriving today - a result directly attributable to the efficacy of the Berlin Heart. The nine survivors ranged in age from six months to 13 years.
Before availability of the Berlin Heart, Walters and his colleagues occasionally adapted an adult ventricular assist device for use in a child, but the results were not optimal and some patients were too small for a modified adult device.
"The Berlin Heart stands alone in filling a tremendous void in the field of pediatric cardiac failure. I can honestly say that of our nine survivors, eight were too small to have possibly accommodated any of the available adult ventricular assist devices. For these patients the Berlin Heart was truly the key to their survival," adds Walters.
The pediatric heart transplant program at the DMC Children's Hospital of Michigan is the largest in the state of Michigan and one of the largest in the Midwest.
For more information: www.childrensdmc.org


 January 15, 2026
January 15, 2026 









