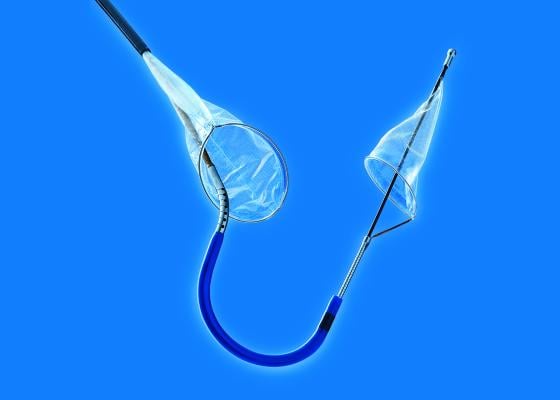
October 7, 2014 — Claret Medical announced the first patient has been successfully treated in its SENTINEL trial, a multicenter pivotal trial of the Sentinel cerebral protection system (CPS). The landmark trial is the first prospective, randomized, controlled, blinded trial in the United States to evaluate the role of cerebral protection during transcatheter aortic valve replacement (TAVR).
The first patient was treated at New York and Presbyterian Hospital/Columbia University Medical Center by Susheel Kodali, M.D., a national co-principal investigator for the trial. The SENTINEL trial will evaluate up to 284 patients at up to 15 centers nationwide.
The primary endpoints for the SENTINEL trial are the reduction in total new lesion volume as determined by diffusion-weighted magnetic resonance imaging (DW-MRI) and major adverse cardiac and cerebrovascular events (MACCE). A number of secondary endpoints, such as neurocognitive and histopathological outcomes during TAVR, will be compared in the study arms with and without cerebral protection.
“Any occurrence of stroke is one too many, and results from this clinical trial may give us the evidence needed to make cerebral protection a standard of care during TAVR, as it is in carotid artery stenting,” said Samir Kapadia, M.D., director of the Cleveland Clinic’s Sones Cardiac Catheterization Laboratories and a national co-principal investigator for the study. “By both capturing and removing embolic debris released during TAVR, the Sentinel CPS may offer a unique neuroprotective benefit. We expect the device to demonstrate a similarly significant reduction in the number and size of lesions in the brains of TAVR patients when cerebral protection is used as was recently reported in the CLEAN-TAVI trial."
At last month’s Transcatheter Cardiovascular Therapeutics (TCT) meeting, 30 day results from the CLEAN-TAVI randomized, controlled trial studying Claret Medical’s cerebral protection system were presented as a late-breaking clinical trial session, demonstrating:
- Fifty-three percent reduction in the total volume of new brain lesions and 60 percent reduction in the number of new brain lesions two days after the TAVR procedure when the Claret Medical cerebral protection system was used;
- Twenty-four percent incidence of the neurological symptoms of ataxia in the control group as compared to nine percent in the treatment group protected with the Claret Medical system in a “per protocol” analysis at two days, which reached statistical significance; and
- Observed neurological deficit in 28 percent of all control patients at two days post-procedure when evaluated by a NIHSS (National Institute of Health Stroke Scale) -trained specialist in an “intent to treat” analysis, demonstrating that prospective assessment pre- and post-procedure can identify more neurological effects than has been reported to date.
Stroke continues to be a devastating complication of TAVR procedures, occurring in approximately 2 to 8 percent of procedures according to published literature. Recently, new ischemic brain lesions, or “silent” infarcts, have been shown to occur in more than 90 percent of TAVR patients. These lesions have been associated with adverse neurologic and cognitive consequences, and dementia. They have also been shown to increase the risk of stroke by two to four times in future years, according to population-based studies published in the 2013 American Stroke Association/American Heart Association consensus guidelines.
For more information: www.claretmedical.com


 January 05, 2026
January 05, 2026 









