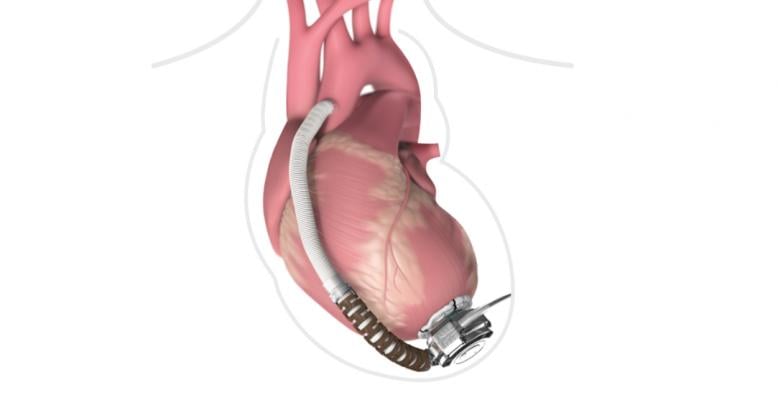
May 29, 2020 — Medtronic is recalling its HeartWare HVAD left ventricular assist device (LVAD) pump outflow graft and outflow graft strain relief because it may tear and the strain relief screw may break during assembly prior to implant. Medtronic said the issues might not be observed until during or after the pre-implant pump assembly and attachment to the HVAD pump.
Medtronic has received 92 complaints related to the pre-implant pump assembly process, which includes both the strain relief screw breaking and outflow graft tears. The recall in the U.S. includes 4,924 devices. The recall was initiated April 3, 2020,
The FDA says this is a Class I recall because use of these affected products may cause serious patient harm including dizziness, loss of consciousness, bleeding, fluid buildup around the heart, additional medical procedures or even death.
The recalled medical products include:
• HVAD Pump Outflow Graft: 1125
• HVAD Pump Implant Kit: 1103
• HVAD Implant Accessories Kit: 1153
The HeartWare HVAD Pump Outflow Graft and Outflow Graft Strain Relief, are parts of the HeartWare Ventricular Assist Device (HVAD) System, that help the heart deliver blood to the rest of the body. The HVAD system is used as a bridge to cardiac transplants in patients who are at risk of death from end-stage left ventricular heart failure, for heart tissue recovery, or as destination therapy (DT) in patients where new transplants are not planned.


 February 03, 2026
February 03, 2026 









