October 23, 2013 — Sunshine Heart Inc. announced its collaboration with Minnetronix for a transcutaneous energy transfer (TET) system to power the company's fully-implantable C-Pulse Heart Assist
System under development. The agreement leverages Minnetronix's existing TET technology and includes milestone payments due to Minnetronix prior to U.S. regulatory approval for the fully-implantable C-Pulse system.
"We are pleased to have entered into our second partnership with Minnetronix," said Dave Rosa, CEO, Sunshine Heart. "Currently, they are the supplier of our second generation C-Pulse driver and we have been pleased with the quality, responsiveness and service they have provided. We look forward to closely partnering with them for our TET system for our fully implantable C-Pulse system."
"Minnetronix is very excited to collaborate with Sunshine Heart on their fully implantable heart assist system," said Rich Nazarian, President and CEO of Minnetronix. "Sunshine Heart's novel technology represents the next generation in chronic circulatory support devices and holds great promise for less invasive and more effective treatment of congestive
heart failure."
Sunshine Heart initiated its COUNTER HF U.S. pivotal trial for its C-Pulse system earlier this year. As announced on Aug. 7, 2013 in conjunction with its second quarter earnings release, the company intends to initiate animal studies for its fully-implantable pump in the fourth quarter of this year. The C-Pulse device is also in an ongoing OPTIONS HF European post-market study.
About the C-Pulse Heart Assist System
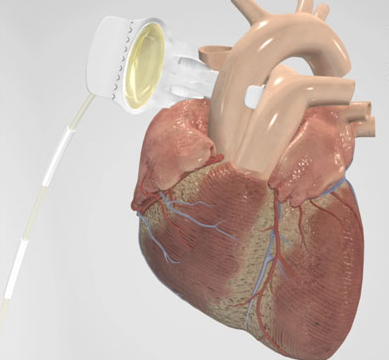
The C-Pulse Heart Assist System, or C-Pulse System, an investigational device in the United States, Canada and countries that do not recognize the CE mark approval, utilizes the scientific principles of intra-aortic balloon counterpulsation applied in an extra-aortic
approach to assist the left ventricle by reducing the workload required to pump blood throughout the body while increasing blood flow to the coronary arteries. Combined, these potential benefits may help sustain the patient's current condition or, in some cases, reverse the heart failure process, thereby potentially preventing the need for later-stage heart failure devices, such as left ventricular assist devices (LVADs), artificial hearts or transplants. It may also provide relief from the symptoms of Class III and ambulatory Class IV heart failure and improve quality of life and cardiac function.
For more information: www.sunshineheart.com, www.minnetronix.com