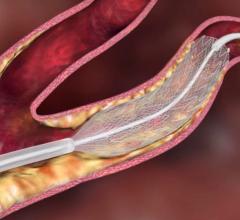
FDA has granted approval to the Cordis Endovascular division of Cordis Corp. to market its PRECISE RX Nitinol Self-Expanding Stent and ANGIOGUARD RX Emboli Capture Guidewire System to treat clogged neck arteries. The approval was announced during the Arizona Heart Institute's International Congress on Endovascular Interventions in Scottsdale, AZ.
The PRECISE RX Nitinol Self-Expanding Stent and ANGIOGUARD RX Emboli Capture Guidewire System differ are from Cordis' over-the-wire carotid system approved last year — the RX, or rapid exchange, version facilitates single operator use and more efficient manipulation of the catheter and guidewire during stenting procedures.
Anil Chhabra, M.D., Willis Knighton Medical Center in Shreveport, LA, performed the first U.S. carotid case with the newly approved carotid stent and emboli protection system.
Cordis Endovascular also announced the initiation of SAPPHIRE Worldwide, a 10,000 patient global registry to assess the 30-day rate of major adverse events (death, stroke or myocardial infarction) following the placement of a stent in high surgical risk patients with carotid artery disease. The stent procedure includes use of an emboli distal protection system — designed to contain and remove plaque or debris from the artery during the procedure.
The registry will include up to 275 centers with low, medium and high annual carotid stent implant volumes, from both academic and private hospitals.

 June 26, 2023
June 26, 2023