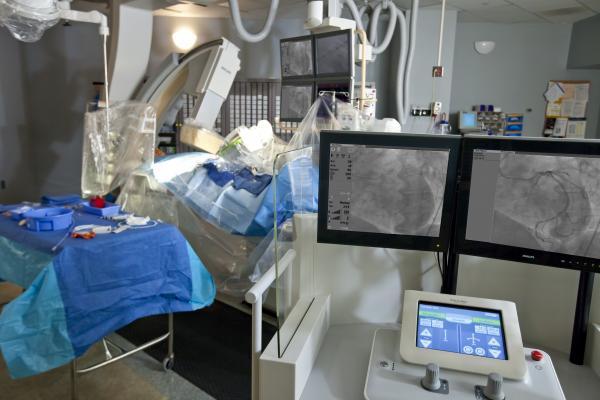
April 12, 2017 — Corindus Vascular Robotics Inc. announced that the first robotic-assisted percutaneous coronary intervention (PCI) procedures were performed in Asia using its CorPath GRX System. One of these procedures was transmitted live from Fu Wai Hospital to the more than 8,000 attendees at the China Interventional Therapeutics Conference. The annual conference, being held in Beijing, China this year, is the largest interventional cardiology conference in the Asia Pacific region.
The CorPath GRX System, placed at Fu Wai Hospital for investigational use, is the first installation of the next-generation CorPath System outside of the United States. Kefei Dou, M.D., deputy director of coronary artery disease for the Department of Cardiology at Fu Wai Hospital, successfully performed the first robotic-assisted PCI procedures in Asia. Fu Wai Hospital is the largest hospital in China and specializes in the treatment, prevention and research of cardiovascular diseases and hypertension and their related complications.
According to Millennium Research Group, China is the second largest PCI market in the world.
"The level of precision that can be attained using the CorPath GRX technology, combined with the potential for remote capabilities in the future, will both expand and improve how we treat our patients," said Xu Bo, MBBS, director of catheterization laboratories, Fu Wai Hospital. "We are honored and excited to be able to participate in the advancement of patient care through robotics and to be the first hospital bringing this technology to Asia."
CorPath GRX offers enhancements to the CorPath platform by adding important key upgrades that increase precision, improve workflow, and extend the capabilities and range of procedures that can be performed robotically. Physicians now have independent and simultaneous robotic control of guide catheters, guidewires and balloon/stent catheters, with 1 millimeter advancement capability, from the workstation console. This precise positioning that allows physicians to adjust guide catheters during PCI procedures may expand the use of the CorPath GRX System to more complex cases.
For more information: www.corindus.com


 November 14, 2025
November 14, 2025 









