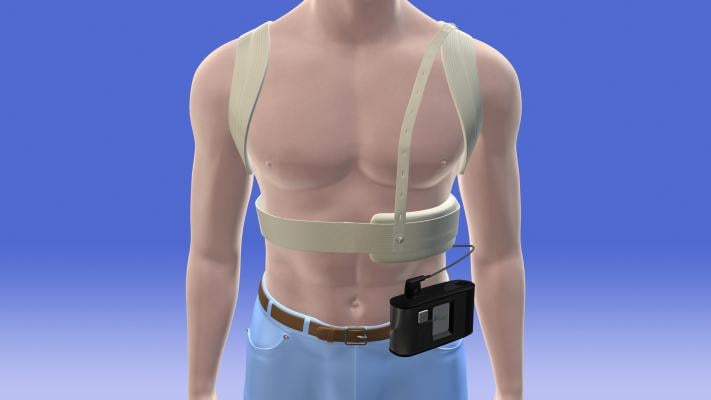
May 1, 2013 — Zoll Medical said results from the prospective registry and follow-up of Patients Using the Wearable Defibrillator (WEARIT-II) trial will be presented in a late-breaking clinical trial presentation at Heart Rhythm 2013. The session will be presented during the Heart Rhythm Society’s 34th Annual Scientific Sessions, Friday, May 10, 1:30-3 p.m., session number SP22.
The 18-month results from WEARIT-II will be presented by Ilan Goldenberg, M.D., (principal investigator) University of Rochester Medical Center. WEARIT-II is a prospective, observational study designed to evaluate high cardiac risk patients’ clinical information, arrhythmias and treatment findings during wearable defibrillator use and subsequent one-year clinical course, including survival.
“As an investigator in the original WEARIT/BIROAD clinical trial and the co-principal investigator for the WEARIT-II Registry, it is rewarding to see that these data will be shared as part of the Late-Breaking Clinical Trial presentations at Heart Rhythm. The LifeVest plays an important role in the continuum of care for patients at high risk from sudden cardiac arrest,” said Arthur J. Moss, M.D., University of Rochester Medical Center. “The LifeVest allows physicians to protect patients during their period of highest risk early after an acute cardiac event such as a myocardial infarction or newly diagnosed nonischemic cardiomyopathy, when other therapies are not appropriate due to the patient’s changing condition and the potential for cardiac recovery.”
“WEARIT-II is the first multi-national registry of LifeVest usage and is currently enrolling patients in the United States, Europe and Israel. We look forward to this presentation of the 18-month U.S. results,” said Marshal Linder, president of Zoll LifeVest. “These data will add to our understanding of the real-world treatment approaches and outcomes from physicians’ utilization of wearable defibrillators.”
The LifeVest Wearable Defibrillator, along with sudden cardiac arrest (SCA) education and screening tools, will be on display in Booth #539 at Heart Rhythm 2013 May 8 to 11 at the Colorado Convention Center in Denver.
For more information: www.zoll.com


 January 05, 2026
January 05, 2026 









