May 2, 2013 — eCardio Diagnostics announced the launch of eCardio Verite — a patient-friendly, wireless monitor offering seamless transition between mobile cardiac telemetry and cardiac event monitoring when directed by a physician.
"We are excited to provide physicians with the innovative new choice in mobile cardiac telemetry through the launch of this monitor," said eCardio's President and Chief Executive Officer Larry Lawson. "With remote programming functionality and built-in symptom and activity capture, eCardio Verite provides physicians with the monitoring services needed to make an accurate arrhythmia diagnosis as quickly as possible."
eCardio Verite is a multi-functional monitor with both event and telemetry monitoring capabilities, including automatic detection, capturing and communication of asymptomatic events such as atrial fibrillation, bradycardia, tachycardia and cardiac pause. A remotely programmable feature of eCardio Verite allows eCardio's monitoring center to seamlessly transition the monitor from a telemetry monitor to an event monitor when directed by a patient's physician without any disruption to a patient's monitoring study.
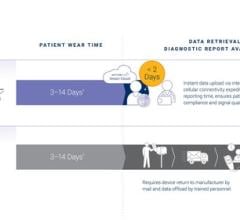
Physicians prescribing eCardio Verite will experience additional benefits through both enhancements to existing reports and a newly developed report, the Verite Initial Assessment. The Verite Initial Assessment is provided for the first 24 hours of monitoring and may alleviate the need for preliminary Holter studies.
"Our new reports for eCardio Verite reflect what physicians have told us is desired to provide optimal care to their patients," said Lawson. "Our nationwide cardiology sales force spends time in practices every day, and those conversations played an integral part in the development of the reports we provide with this new monitor."
The enhanced reports highlight the data output from the monitor's advanced algorithm and also provide the data in organized snapshots that include a daily summary, a five-day summary and a full-study summary of each arrhythmia by type and by date.
For more information: www.ecardio.com


 March 31, 2025
March 31, 2025