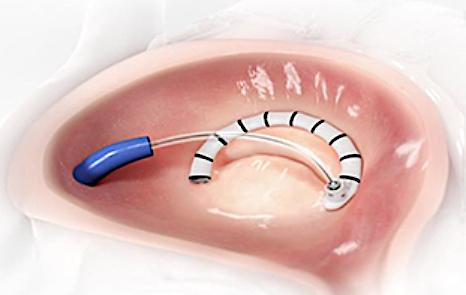
The Edwards Lifesciences Cardioband for tricuspid valve regurgitation is one of the device trials being paused for the duration of the COVID-19 outbreak.
March 30, 2020 — Edwards Lifesciences Corp. announced it is temporarily pausing new enrollments in its active pivotal clinical trials of transcatheter mitral and tricuspid therapies. This is in response to the urgent novel coronavirus (COVID-19) response around the globe.
Edwards is coordinating closely with the trials' clinical investigators, and the decision to resume enrollments in the trials will be made in consultation with each investigator and hospital at the time when their clinicians' and patients' needs can be better served.
Many hospitals in the United States have postponed all non-essential tests and procedures until further notice in an attempt to conserve personal protective equipment (PPE) and help contain the rapid spread of the virus. Read more in the article CMS Calls For Postponing All Elective Cardiac Tests and Procedures to Aid COVID-19 Containment.
Among the trials that are being paused is the Transcatheter Repair of Tricuspid Regurgitation With Edwards Cardioband TR System Post Market Study (TriBAND).
Edwards said the analyses from the CLASP TR Early Feasibility Study was presented at the virtual American College of Cardiology (ACC) meeting at https://virtual.acc.org/.


 January 05, 2026
January 05, 2026 









