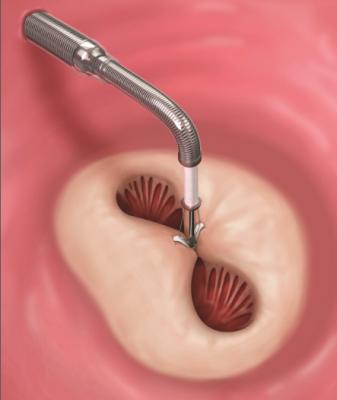
A MitraClip device being deployed to clasp together the leaflets of the mitral valve. This mimics a surgical suture repair to create a double orifice valve with better leaflet coaptation to prevent mitral regurgitation.
May 11, 2018 — The post-approval study evaluating the safety and efficacy of MitraClip in a real-world, commercial setting was presented as late-breaking clinical science at the recent 2018 Society for Cardiovascular Angiography and Interventions (SCAI) 2018 Scientific Sessions. The prospective, single-arm, multi-center, observational study entitled, "The Abbott Post-Approval Study 1 MitraClip Registry: 1-year Results of the First 2,000 Patients in the Transcatheter Valve Therapy Registry," is the first to include echocardiographic, functional and quality of life outcomes.
Nearly 10 percent of people 75 years or older are subject to mitral valve regurgitation (JAHA). In 2013, the MitraClip System became the U.S. Federal Drug Administration's (FDA) first commercially approved alternative to mitral valve regurgitation surgery, creating a much less invasive way to treat the condition (FDA). In the US, MitraClip, developed by healthcare company Abbott, is currently indicated for mitral regurgitation (MR) patients who experience degenerative mitral valve regurgitation, caused by a leaking heart valve that allows blood to flow backward in the heart. Left untreated, MR can place serious burdens on day-to-day activities for patients and may contribute to a variety of serious complications, including heart failure. MitraClip provides a minimally invasive treatment option for patients for whom traditional surgery is not an option due to comorbidities or frailty.
At one-year, 86.8 percent of patients had post-procedural MR of ≤2+ and there was 81.7 percent freedom from all-cause mortality. Measurements of the left ventricle by echocardiogram showed improvement in left ventricular end-diastolic volume (LVEDV) and left ventricular internal diameter end diastole and end systole (LVIDd) with 8.5 ml and 0.2 cm mean reduction, respectively. Clinical measures show mean improvement of 37.9 m in the six-minute walk test and 83.4 percent improvement with NYHA I/II at the one-year mark.
"This is a rigorous, real-world study that includes more data than we've seen before. For the first time, we evaluated results of an echocardiogram and walk test, and assessed the patient's quality of life to further evaluate the effectiveness of MitraClip therapy," said lead author James Hermiller, M.D., MSCAI, St Vincent Heart Center of Indiana, Indianapolis IN. "The outcomes were extremely encouraging and reinforce the safety and efficacy of this treatment option in real world practice. We think it has the potential to be a new standard of care for select patients with mitral valve regurgitation."
The study is based on data extracted from the first 2,000 MitraClip patients consecutively entered into the National Transcatheter Valve Therapy Registry (TVT Registry), which is housed jointly by the American College of Cardiology Foundation (ACCF) and the Society for Thoracic Surgeons (STS). Clinical, echocardiographic, functional and quality of life outcomes were analyzed at 30-days and one-year after the device was implanted. The study included a patient population that was 56.5 percent male and a mean age of 79±10 years. Additionally, 85.6 percent of patients on the New York Heart Association (NYHA) scale classified as class III/IV, with 90 percent indicated as prohibitive risk at baseline.
The authors of this study note an additional randomized study being conducted in the field of mitral valve regurgitation which will further study the MitraClip device in symptomatic functional mitral valve regurgitation (FMR) patients with heart failure, called the COAPT study. Primary results from the study are expected to be shared in 2018 and, if successful, will provide evidence for expanded indication for MitraClip to treat FMR. Degenerative mitral regurgitation (DMR) is caused by abnormality of valve structures including the valve leaflets, valve ring, and chordae tendineae. Functional mitral regurgitation (FMR) is a disease that occurs when the left ventricle of the heart dilates, leading to incomplete coaptation of the mitral valve.
The STS/ACC TVT Registry is a benchmarking tool developed to track patient safety and real-world outcomes related to transcatheter valve replacement and repair procedures and emerging treatments for valve disease patients. Created by The Society of Thoracic Surgeons (STS) and the American College of Cardiology, the TVT Registry is designed to monitor the safety and efficacy of these new technologies for the treatment of valve disease. Through the capture and reporting of patient demographics, procedure details, and facility and physician information, the TVT Registry provides a data repository capable of delivering insight into clinical practice patterns and patient outcomes.
Complete listing of SCAI 2018 late-breaking trials with links to articles.
Read the related article Treating Mitral Regurgitation in High Risk PatientsUsing the MitraClip — article by Allen Atchley, M.D.
For more information: www.vascular.abbott/us/products/structural-heart/mitraclip-mitral-valve-repair.html


 November 14, 2025
November 14, 2025 









