
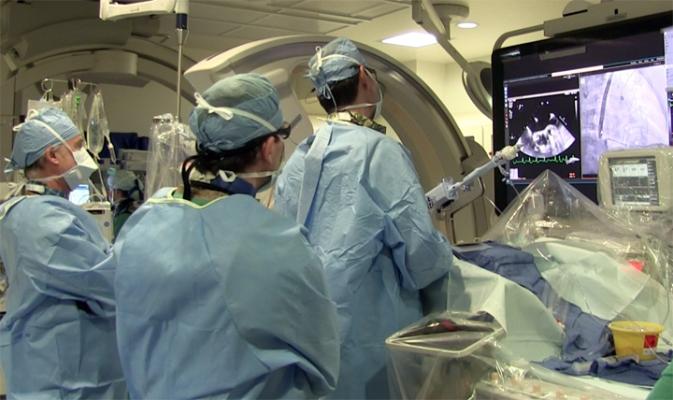
The implantation of a MitraClip is guided under live transesophageal echo (TEE). This image shows the operator moving the open clip into position and engaging the mitral valve leaflets. The clip appears as an arrowhead shape in the ultrasound with the leaflets coming off each side. Photo from a MitraClip procedure at the University of Colorado Hospital.
Mitral regurgitation (MR) is one of the most common types of heart valve diseases in the United States, affecting approximately one in 10 adults age 75 and older. About 3 to 4 percent of the population has moderate to severe degenerative MR. It is a progressive disease that, without treatment, leads to a cascade of events that typically progress to heart failure and death.[1]
Among the estimated 4 million people who suffer from MR in the United States, about 50,000 undergo surgery annually. But many other patients are denied surgery due to the presence of significant comorbidities, which places them at high risk for surgical complications. With limited options, these patients often suffer from slowly deteriorating health because medical therapy can only manage their symptoms, not address the underlying cause.
For symptomatic patients with moderate-to-severe MR, surgery is generally recommended to repair or replace the mitral valve. Open-heart surgery with cardiopulmonary bypass is required, and patients typically take six to 12 weeks to regain their previous level of physical function and activity.
The advent of a less invasive alternative – transcatheter mitral valve repair for patients who previously had no other options – is bringing cardiologists and surgeons together as multi-disciplinary teams to discuss challenging cases and potential treatment options.
Fortunately, the availability of minimally invasive therapies have surgeons and cardiologists starting to think differently. With the advent of MitraClip, a first-of-its-kind transcatheter mitral valve repair system approved by the U.S. Food and Drug Administration (FDA) in 2013, we have a more aggressive and effective treatment for patients who have been deemed too high-risk for mitral valve surgery.
Watch the VIDEO: How to Implant the MitraClip Transcatheter Mitral Valve Repair Device.
Real-world Experience With MitraClip
The 2014 American Heart Association (AHA)/American College of Cardiology (ACC) Guideline for the Management of Patients with Valvular Heart Disease states that transcatheter mitral valve repair with MitraClip therapy may be considered for patients with severe degenerative MR who are not suitable for surgery.
Some of the most memorable endorsements come from our experience with patients.
We helped take care of a 96-year-old retired war hero who had flown several hundred missions, both in World War II and in Korea. He was experiencing activity-limiting exertional shortness of breath and an echocardiogram, ordered by his primary care physician for a worsening murmur, revealed severe mitral regurgitation. His posterior leaflet was torn (flail). This structural anatomic problem cannot fix itself and cannot be adequately treated with medication. The surgeon assessed the gentleman as too high risk for surgery due to his advanced age and other medical problems, so he was referred to our lab for further evaluation and possible transcatheter intervention.
We did a right and left heart catheterization, transesophageal echo (TEE), and he completed a six-minute walk test. His care was then discussed among our multi-disciplinary structural heart team, which determined that he was a good candidate for the MitraClip procedure.
After his procedure, which took about two and a half hours, his severe (grade 4+) MR was significantly improved to only trace-mild (1+) residual regurgitation. He got up and walked out of the hospital the next day, and was then able to travel for an out-of-town ceremony honoring his service to the country soon thereafter.
This is an example of what makes transcatheter mitral valve repair so rewarding. This patient came to us with no other options and would have developed progressive shortness of breath and heart failure symptoms over time. The MitraClip procedure gave him the ability to enjoy a far better quality of life.
Mitral Regurgitation is Frequently Under Treated
MR is often untreated. Only about 2 percent of the estimated 1.7 million patients with moderate-severe or severe MR (greater than or equal to 3+) are treated surgically. However, the paradigm for treating MR has shifted over the past decade, and physicians now recognize the need for more aggressive procedural intervention. Traditionally, cardiologists and surgeons would wait for people to have symptoms, such as shortness of breath and activity intolerance, or structural changes in the heart before recommending surgery. While medical management can help relieve symptoms, cardiologists and surgeons know that if a patient has degenerative mitral valve disease, and it’s possible to repair their valve with 95 percent certainty, then that patient should get a repair to avoid irreversible changes.
Why are many MR patients denied surgery? Issues include advanced age, frailty, prior open-heart or chest surgeries, arrhythmias, chronic kidney disease and other factors.
Transcatheter mitral valve repair has expanded our horizons for how we can treat patients. While surgical repair remains the standard, this alternative provides an option for those who are not candidates for surgery or who previously had no options at all.
But MitraClip has done more than just add to our treatment armamentarium. It is changing the way we think about MR and how to properly treat patients. The device has initiated a new dialogue between patients, surgeons and cardiologists, and is allowing healthcare teams to better treat older and more complex patients.
The Burden of Mitral Regurgitation
A variety of factors contribute to the burden of MR — it is a costly and morbid condition than can cause chest pain/discomfort, palpitations and arrhythmias, congestive heart failure, repeat hospitalizations and even death.
Watchful waiting is not appropriate when dealing with moderate or severe MR, especially now that we have a treatment option to repair a mechanical problem that cannot be fixed with medicine alone.
A Quick Primer About Mitral Regurgitation
The mitral valve annulus has a complex, saddle-like shape that changes throughout systole and diastole to facilitate proper valve function. The valve leaflets are suspended from the annulus and connect via chordae tendineae to the papillary muscles and left ventricle.
The etiology of the two types of MR – degenerative and functional – are often mixed. Primary or degenerative MR is due to the predisposition to a loss of fibro-elasticity in the valve tissue, which is also called myxomatous degeneration. This results in diseased leaflets with excess tissue/motion. The resulting MR can then lead to further valve deterioration, including torn chordae (flail segments) and chamber enlargement, which leads to worsening MR.
In functional, or secondary MR, the valve leaflets demonstrate normal or near-normal tissue and mobility with MR, occurring due to other conditions that can affect mitral valve function. Left ventricular dysfunction from ischemic heart disease can restrict leaflet mobility, causing malcoaptation and MR. Chamber enlargement, from a dilated cardiomyopathy or left atrial enlargement, can lead to annular dilation and incomplete coaptation of the mitral leaflets as the cause of regurgitation. In functional MR, although the valve leaflets are essentially normal, other abnormalities in heart structure or function result in mitral valve dysfunction.
There is also calcific mitral degeneration, or rheumatic MR, which is characterized by progressive leaflet thickening, calcium deposition, and subsequent leaflet tethering and restricted motion.
Another way to define MR has been developed by Alain Carpentier M.D. Ph.D., Hopital Europeen Georges Pompidou, Paris, whose classification system includes components of etiology; lesions which result from the disease; and dysfunctions which result from the lesions:
• Type I — Normal leaflet motion, a dilated annulus and central MR
• Type II — Excess leaflet motion (myxomatosis changes and leaflet prolapse/flail)
• Type IIIa — Restricted leaflet opening (typically Rheumatic and calcific disease)
• Type IIIb — Restricted leaflet mobility and closure (LV dysfunction and ischemic disease)
These distinctions are important because prognosis may ultimately be dependent on the underlying cause of the problem. Treatment strategies and surgical approach rely on the etiology of MR and lesions. It should also be noted that the etiology of MR can “mixed,” resulting from both degenerative and functional components. MitraClip is currently used for degenerative MR. However, the vast majority (more than two-thirds) of people diagnosed with MR have functional MR. Untreated, moderate-severe and severe MR can lead to left atrial dilation, permanent atrial fibrillation, left ventricular enlargement, shortness of breath, and congestive heart failure.
Abbott's COAPT Trial,[5] which is the first randomized, controlled, multi-center study designed to evaluate the safety and efficacy of MitraClip for heart failure patients with functional MR, has results presented at the 2018 Transcatheter Cardiovascular Therapeutics (TCT) meeting. The data showed overwhelmingly that using of the clip procedure improved patient outcomes and reduced heart failure readmissions.
Read more on the results of the COAPT Trial.
Watch the VIDEO: MitraClip to Treat Heart Failure - Results of the COAPT Trial — an interview with William Abraham, M.D., FACC, professor of medicine and director of the division of cardiovascular medicine, The Ohio State University Wexner Medical Center
Watch the VIDEO: Impact of the COAPT Trial on Heart Failure Patients With Functional Mitral Regurgitation — an interview with Andreas Brieke, M.D., director of mechanical circulatory support, heart failure physician and site principal investigator for the COAPT Trial at the University of Colorado Hospital,
A Transcatheter Alternative to Open Surgery
MitraClip provides transcatheter mitral valve repair by creating a vertical line of coaptation, forming a double-orifice valve. It is a beating-heart procedure with no cardiopulmonary bypass required. The procedure allows for real-time positioning and repositioning to optimize the reduction of mitral regurgitation.
The MitraClip procedure is performed by a team including a cardiac surgeon, an interventional or structural cardiologist with experience in transseptal puncture, a cardiac anesthesiologist, an echocardiographer who specializes in transthoracic echo (TTE), and trained OR or cath lab staff.
Once implanted, MitraClip mimics a type of surgical repair “stitch” performed during open-heart and valve repair surgery. The result is the heart’s ability to pump blood more efficiently, thereby relieving symptoms, improving the patient’s quality-of-life and allowing them to get back to being active- faster.
The median hospital stay is about 2.5 days. Even patients with severe left ventricular dysfunction typically tolerate the procedure well.
Cost/Benefit to Hospitals and Healthcare Organizations
It is important to have institutional support both monetarily and administratively. Appropriate training is necessary and registry monitoring is required. The team at the Chattanooga Heart Institute started planning a year before our first procedure, so there is a time commitment.
However, the procedure itself can be profitable. With experience, the team becomes better at patient selection, which helps to reduce procedural time and find efficiencies in patient care. Offering the MitraClip procedure tends to attract patients to your institution, and some patients are referred to surgeons.
Clinically Significant Results From Transcatheter Mitral Valve Repair
More than 65,000 patients have received the MitraClip procedure worldwide. The major clinical benefits of MitraClip are reduction of MR to <2+, which can reduce hospitalizations, improve quality of life, reverse left ventricular remodeling and provide symptomatic relief in patients who have no other therapeutic options.
In March 2017, a study by Paul Sorajja, M.D., Minneapolis Heart Institute, presented at the American College of Cardiology’s 66th Annual Scientific Session, showed favorable one-year outcomes for the MitraClip system in transcatheter mitral valve repair procedures in the United States. The study analyzed data from the Transcatheter Valve Therapy (TVT) registry involving almost 3,000 patients who were high risk for surgery. After implantation of the MitraClip, 92.8 percent of patients achieved post-procedural MR severity gradient of <2. In addition, 80 percent of patients remained free from heart failure re-hospitalization in the year post hospitalization.
Similarly, a study by James Hermiller, M.D., St. Vincent Heart Center of Indiana, presented at the Society for Cardiovascular Angiography and Interventions (SCAI) 2018 Scientific Sessions, demonstrated an 81.7 percent freedom from all-cause mortality at one year and 83.4 percent improvement with NYHA I/II at the one-year mark after treatment with MitraClip. In addition, there was mean improvement of 37.9 meters in the six-minute walk test.
MitraClip has shown consistent reduction in MR and associated heart failure. The procedure has a high procedural success rate and a low incidence of complications. Patients have notable improvement in symptoms and a reduction in hospital stays for heart failure. For instance (from the Journal of the American College of Cardiology, March 7, 2016, Paul Sorajja, M.D.):
• 90.6 percent of patients had procedural success
• 8 percent had procedural complications
• 3.9 percent had major bleeding
• 1.4 percent had device-related adverse events
• No evidence of development of an acute low-output state after implantation
• Four-year follow-up of patients (in the EVEREST II trial) has shown no increase in late MR recurrence compared with surgery
More Aggressive Evaluation, Treatment of MR in Non-Surgical Patients
MitraClip is increasing our understanding and awareness of MR and the need for more aggressive evaluation and treatment of MR in patients who are not surgical candidates and have previously been considered virtually untreatable. We have learned that many patients who have been presumed to be non-operative candidates will do well with the percutaneous procedure. We are also learning more about MR and how it can best be treated. While surgery continues to be the gold standard of care, transcatheter mitral valve repair offers hope for patients who are too high risk for surgical intervention.
Editor's Note: Allen Atchley, M.D., FACC, is a cardiologist at The Chattanooga Heart Institute. He specializes in interventional cardiology, echocardiography and nuclear cardiography.
Related MitraClip Content:
VIDEO: Mitral Valve Repair and Replacement Technologies — Interview with Ted Feldman, M.D.
Expanded MitraClip Registry Shows Continued Positive Results
VIDEO: Evolution of Transcatheter Mitral Valve Repair at the University of Colorado — Interview with John Carroll, M.D., and Robert Quaife, M.D.
Abbott Receives FDA Approval for Third-Generation MitraClip Device
MitraClip Reduces Mortality for Heart Failure Patients With Secondary Mitral Regurgitation
VIDEO: MitraClip to Treat Heart Failure - Results of the COAPT Trial — Interview with William Abraham, M.D.
VIDEO: Overview of Transcatheter Mitral Valve Repair Technologies at TVT 2015 — Interview with Ted Feldman, M.D.
VIDEO: The Role of Advanced Imaging in Structural Heart Interventions
References:
1. Nkomo VT, Gardin JM, Skelton TN, Gottdiener JS, Scott CG, Enriquez-Sarano M. Burden of valvular heart diseases: a population-based study. Lancet. 2006 Sep 16;368(9540):1005-11. https://www.ncbi.nlm.nih.gov/pubmed/16980116
2. Patient Info. Mitral Regurgitation. 2017. Accessed July 25, 2018 at: https://patient.info/health/mitral-regurgitation-leaflet.
3. National Heart, Lung and Blood Institute. What is Heart Valve Disease? 2015. Accessed June 19, 2018 at: https://hhlbi.nih.gov/health/health-topics/hvd.
4. Dang NC. Aboodi MS, Sakaguchi T, Wasserman HS, Argenziano M, Cosgrove DM, Rosengart TK, Feldman T, Block PC, Oz MC. Surgical revision after percutaneous mitral valve repair with a clip: initial multicenter experience, Ann Thorac Surg. 2005 Dec; 80(6):2338-42.





 November 14, 2025
November 14, 2025 









