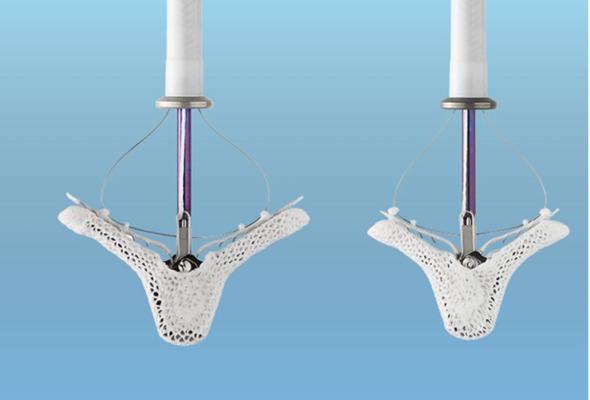
September 9, 2022 — The U.S. Food and Drug Administration (FDA) is alerting healthcare providers about potential clip lock malfunctions with MitraClip Clip Delivery Systems manufactured by Abbott.
On September 8, 2022, Abbott issued an Urgent Medical Device CorrectionExternal Link Disclaimer to inform healthcare providers about the issue. An increased rate of reports of clip lock malfunctions has been observed before and after clip deployment. These events appear to occur in approximately 1.3% of MitraClip procedures and have been observed with all device models.
The potential risk to patients in the event of a clip lock malfunction includes ineffective treatment of mitral regurgitation (MR) and the potential need for additional interventions contributing to increased procedural risks such as bleeding, complications with implanting additional clips, and longer procedural times. The majority of reported clip lock malfunction events have not been associated with adverse patient outcomes. Based on the available data on clip lock malfunctions and the associated risks, the FDA believes that the probable benefits of the MitraClip device continue to outweigh the probable risks for the approved indications for use. The FDA is issuing this letter to ensure you are aware of the manufacturer's recall notice and recommended actions.
Recommendations
The FDA recommends that health care providers:
- Review the recall noticeExternal Link Disclaimer from Abbott for all MitraClip Clip Delivery Systems
- Be aware of the potential for clip lock malfunctions before or after deployment with this device
- Read and carefully follow the Instructions for Use and the recommendations provided in Abbott's recall noticeExternal Link Disclaimer to help minimize the chance of the clip failing to lock. These include recommendations about procedural steps for implant positioning, locking sequences, establishing clip arm angle, preparation for clip release, and avoiding excessive force and manipulation when unlocking the clip during device preparation and during the procedure.
- Report any adverse events or suspected adverse events with MitraClip to the FDA, including events of clip lock malfunction. Refer to the Reporting Problems to the FDA section below.
Background
The MitraClip Clip Delivery System is a heart valve repair device that is intended to treat MR. MitraClip was first approved in 2013 to reduce MR in selected patients:
- whose significant symptomatic MR and heart failure symptoms result from abnormalities of the mitral valve (commonly known as primary or degenerative MR); and
- whose risks for mitral valve surgery are prohibitive.
In 2019, a new indication for the device was approved to include treatment of patients with structurally normal mitral valves who develop heart failure symptoms and moderate-to-severe or severe MR due to left heart enlargement and diminished function (commonly known as secondary or functional MR) despite treatment with optimal medical therapy.
MitraClip is currently the only percutaneous (implanted through the skin without open surgery) repair device approved in the United States to treat patients with MR.
FDA Actions
The FDA is working with the manufacturer to continue to evaluate reports of clip lock malfunctions and identify other potential contributing factors and mitigation strategies. The FDA will continue to monitor reports of adverse events related to the issue.
The FDA will keep health care providers and the public informed if new or additional information becomes available.
Reporting Problems to the FDA
The FDA encourages health care providers to report any adverse events or suspected adverse events experienced with Abbott MitraClip Delivery Systems.
- Voluntary reports can be submitted through MedWatch, the FDA Safety Information and Adverse Event Reporting program.
- Device manufacturers and user facilities must comply with the applicable Medical Device Reporting (MDR) regulations.
- Health care personnel employed by facilities that are subject to the FDA's user facility reporting requirements should follow the reporting procedures established by their facilities.
Prompt reporting of adverse events can help the FDA identify and better understand the risks associated with medical devices.


 July 08, 2024
July 08, 2024 









