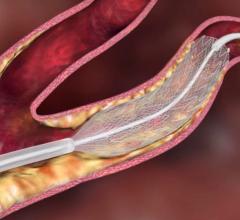
The FDA has cleared Boston Scientific’s NexStent Carotid Stent and Monorail Delivery System for use in patients with carotid artery disease who are at high risk for surgery. The NexStent Carotid Stent is manufactured by EndoTex Interventional Systems, Inc. and has been distributed exclusively by Boston Scientific outside the U.S. since receiving European CE Mark in 2005.
Boston Scientific is slated to acquire EndoTex within 90 days under the terms of the companies' existing agreements. BSI says its FilterWire EZ Embolic Protection System, which was studied together with the NexStent Carotid Stent in the CABERNET clinical trial, is still pending 510(k) clearance by the FDA.
"This is an innovative stenting system that interventionalists in the U.S. have been waiting for," said Subbarao Myla, M.D., co-principal investigator of the CABERNET Trial, of Hoag Memorial Hospital in Newport Beach, CA. "Study results demonstrate excellent outcomes at one month — when there is the greatest risk of procedure-related stroke — through one year in patients with a wide range of lesions and vessel anatomy."
The NexStent Carotid Stent is a laser-cut, nitinol stent with a rolled sheet design that enables one stent size to adapt to multiple diameters in tapered or non-tapered vessel configurations. Its self-sizing feature is designed to provide adaptability when treating lesions in the carotid arteries, and its closed-cell configuration is designed to increase lesion coverage and provide a smooth inner lumen to help facilitate delivery and retrieval of ancillary devices
For more information, visit www.bostonscientific.com.

 June 26, 2023
June 26, 2023